Vaginal-assisted laparoscopic sacrohystero/colpopexy with retroperitoneal tunneling: tips and tricks, and a review of the literature
Introduction
Pelvic organ prolapse (POP) is a significant healthcare problem, affecting millions of women throughout the world. POP is defined as the descent of one or more pelvic organs as a result of vaginal support defects of the anterior, posterior, or apical vaginal vault which might include uterine prolapse (1). The lifetime risk of women to undergo a single operation for POP is about 11%, and about 30% will undergo repeat surgery (2). The surgical techniques available for POP can be categorized as vaginal, abdominal, and laparoscopic (conventional or robotic) approaches.
Following today’s evidence, the sacral hystero/colpopexy (SH/C) procedure is accepted as the “gold standard” in apical prolapse surgery (3,4), and the laparoscopic approach should be preferred due to the superiority of minimally invasive surgery (MIS) (5). However, laparoscopic sacrohystero/colpopexy requires high levels of laparoscopic operative skills and experience and is associated with longer operative times (2,6). For this reason, we designed our novel technique, which combines the vaginal and laparoscopic approaches and incorporates retroperitoneal tunneling; vaginal-assisted laparoscopic sacrohystero/colpopexy (VALSH/C) with retroperitoneal tunneling. This technique makes the operation expressively easy and significantly shortens the operation time. First, in October 2016, we presented our technique at the annual meeting of the Turkish Society of Obstetrics and Gynecology and received the best video presentation award (7). Then, our technique was published in the International Journal of Gynaecology and Obstetrics in October 2017 (8). Since then, we have performed this new technique in more than 20 patients and developed crucial tips and tricks.
Here, we aim to explain these tips and tricks that were important in the implementation of the procedure. Since a step-by-step explanation (video article) of the technique has already been presented (8), only tips and tricks will be described here. Also, the literature was reviewed, and we summarize the current evidence on VALSH/C to determine its outcomes and utilities.
Data sources
A database Google Scholar, Cochrane Library, Scopus, and PubMed/Medline literature search was conducted for all articles published in English from January 1994 to May 2020 using the keywords; “laparoscopic sacrohysteropexy”, “laparoscopic sacrocolpopexy”, “vaginal assisted laparoscopic sacrohysteropexy”, “vaginal assisted laparoscopic sacrocolpopexy”, “retroperitoneal tunneling”, “new technique”, “combining vaginal and laparoscopic approaches”, “VALSH”, and “VALSC”. Duplicate titles and abstracts were removed and the remaining studies were selected according to relevance. Articles were selected based on the following criteria: English language, SH/C procedure performed by combining the vaginal and laparoscopic approaches. Two authors (OL Tapisiz, S Kiykac Altinbas) assessed the selected full-text papers and those that did not meet the inclusion criteria were excluded. Finally, chosen papers were evaluated rigorously to determine VALSH/C procedure outcomes and utilities.
Tips and tricks for VALSH/C with retroperitoneal tunneling technique
This section is described according to VALSH, and all steps can be easily adapted to the VALSC procedure.
Preoperative period
- In our clinical practice, we prefer the patient to be under the age of 45, to have completed her fertility, and have no desire to conceive;
- The patient should be evaluated in detail in terms of pelvic infections in the preoperative period. If there is a suspicious condition or finding in terms of pelvic infection, the operation should be postponed, and the patient should receive systemic plus local antibiotic therapy for 14 days. Then, the patient should be re-evaluated, and operated when the infection regresses;
- The patient should receive sequential compression devices and subcutaneous low molecular weight heparin in both the peri and intraoperative periods.
Intra-operative period
- All surgical steps should be performed with a foley catheter in the bladder;
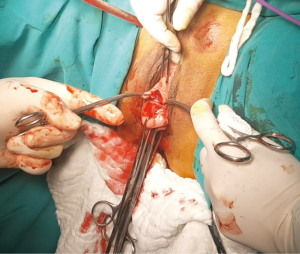
- The small semilunar incisions (3 cm) are made in the upper and lower lip of the cervix during upward and downward traction applied to the cervix via the Jacobs tenacula (Figure 1);
- The bladder and the rectum should be dissected very gently, and a Deaver retractor should be used at these stages;
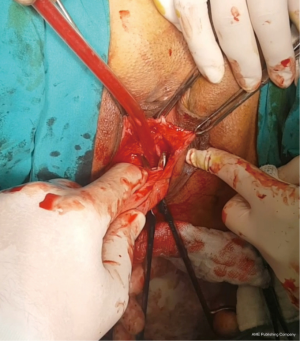
- In the cervical tunneling step, where the tunnel is planned to be opened on the sidewalls of the cervix, the vaginal mucosa should be thick enough to prevent mesh erosion, and caution should be taken against possible uterine artery injury (Figure 2);
- The polypropylene, monofilament, synthetic macroporous mesh (Y-shaped) should be used;
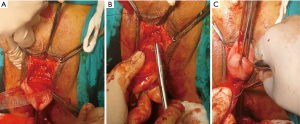
- The short arms of the mesh should be transferred from the tunnels on the sidewalls of the cervix and attached to both the anterior and posterior part of the cervix with a delayed absorbable suture (preferably 2/0) by three/three-knot tying (Figure 3A,B,C);
- The vaginal submucosal suturing should be performed on top of the mesh to prevent mesh erosion;
- The vaginal mucosa should be closed with interrupted sutures. This approach reduces the risk of mesh erosion;
- The peritoneal entry should be avoided as much as possible during the whole vaginal surgery. If entered into the peritoneum, it must be carefully closed before proceeding to laparoscopy to help to maintain the pneumoperitoneum;
- In the whole process, care should be taken to avoid bacterial contamination from the anal region. To achieve this, the anal orifice should be covered with a self-adhesive perineal drape during the procedure. Failure to do this could lead to postoperative fever, undesirable healing, mesh erosion, and abscess formation;
- The sigmoid colon should be hung on the anterior abdominal wall for adequate surgical exploration during the laparoscopic surgery;
- The peritoneum over the sacral promontory is opened with a 3-cm incision and retroperitoneal tunneling is performed downwards with the help of a grasper. At this step, injury to the right ureter and sigmoid colon should be avoided;
- The vaginal surgeon opens the path with his/her index finger behind the uterus without entering the peritoneum and places the Winter Ovum Forceps (blunt/sharp, fine-tipped) on the path formed by the index finger;
- The vaginal surgeon introduces the Winter Ovum Forceps upward to the promontory via retroperitoneal tunneling with the laparoscopic surgeon’s guidance until the connection of the tunnel with the sacral incision. Since the image is inverted on the laparoscopic camera, to prevent complications, this process should be continued only by the laparoscopic surgeon’s guidance;
- The free suture thread (USP size 1) is inserted into the abdomen through the laparoscopic 5-mm right port site, the Winter Ovum Forceps grasps and pulls it to the vaginal region, and the thread is stitched to the long arm of the mesh. When the opposite tip of the thread is pulled from the right port site, the long arm of the mesh turns and transfers to the sacral promontory by retroperitoneal tunneling (Figure 4A,B,C);
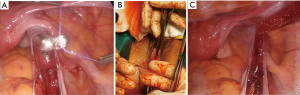 Figure 4 Transferring process of the mesh to the sacral promontory. (A) Grasping the free suture thread and pulling it to the vaginal region with the Winter Ovum Forceps; (B) stitching the free suture thread to the long arm of the mesh; (C) transferring the long arm of the mesh to the sacral promontory via retroperitoneal tunneling.
Figure 4 Transferring process of the mesh to the sacral promontory. (A) Grasping the free suture thread and pulling it to the vaginal region with the Winter Ovum Forceps; (B) stitching the free suture thread to the long arm of the mesh; (C) transferring the long arm of the mesh to the sacral promontory via retroperitoneal tunneling. - The mesh should be copiously irrigated with serum physiologic (SF) before being placed into the peritoneal cavity (macropores should be free of coagulum);
- The level of the cervix should be adjusted anatomically at 2 to 3 cm less than total vaginal length. The mesh should be fully flattened, without excessive tension and in accordance with the curve of the pelvic cavity;
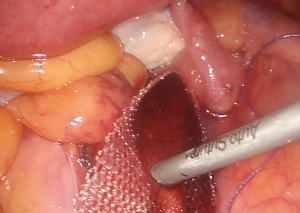
- After cervical anatomic adjustment, the mesh is anchored to the anterior longitudinal ligament of the sacral promontory using three 5-mm helicoidal titanium tacks. The tacker should not be applied forcefully on the sacral promontory due to the risk of slipping. It should be kept in mind that the vital anterior sacral blood vessels can be injured during the process (Figure 5);
- To follow and eliminate the bleeding, the peritoneum on the sacral promontory is closed after all other procedures are completed.
Postoperative period
- Early patient mobilization should be started as soon as possible;
- Antibiotic therapy is administered for the first 5 days postoperation;
- Patients are evaluated on the seventh postoperative day, and at the 1-, 3-, and 6-month follow-ups;
- Weight lifting and sexual intercourse should be restricted for 3 months postoperatively.
Discussion
Abdominal SH/C has long been regarded as the “gold standard” procedure for apical prolapse due to its superiority in anatomic durability (3,4,9) and MIS, through either a standard laparoscopic (LSH/C) or robotic-assisted approach should be preferred due to the shorter recovery time, reduction in complication rate and reduced pain (5,10). Although LSH/C requires a high level of laparoscopic experience and suture knowledge, the VALSH/C technique eliminates these requirements. In this article, we have presented the tips and tricks gained through our experience of the VALSH/C technique. This should help to achieve standardization of the procedure, thereby maximizing compatibility, interoperability, safety, repeatability, and quality.
The learning curve for LSH/C has been relatively long (11), likely due to the laparoscopic skill set required to perform dissection, suturing, and knot tying. Claerhout et al. stated that operating times plateaued after 90 cases, and complications and failures were minimized after 60 cases (12). The other studies performed by Mustafa et al. (13) and Akladios et al. (14) reported that 30–40 and 18–24 cases were necessary for acquiring tips and improving the level of comfort and expertise, respectively. We believe that this long learning curve period can be overcome with the VALSH/C procedure. The most challenging part of the LSH/C procedure, the suturing process, has been handled via the vaginal approach, not opening the entire pelvic peritoneum due to retroperitoneal transfer of the mesh, and anchoring the mesh to the sacral promontory using a tacker, thereby reducing the learning curve significantly.
In the literature, the operation time for LSH/C at the completion of the learning curve was defined as 160–240 min (12-15) and, in general, the average length of the surgery was reported as 158 min, with extremes of 96 and 286 min (16). With our technique, the average operation time was significantly reduced (to 60–100 min), similar to the results of other VALSH/C studies (8,17-25). In our opinion, this reduction should be taken into consideration for eliminating the complications that might occur due to prolonged operation times (e.g., anaesthetic, pulmonary, and thromboembolic complications). It should be noted that this condition will be especially important for elderly patients.
We have performed VALSH/C with retroperitoneal tunneling in more than 20 patients and have encountered no major complication in either the intra or postoperative period. Only one ~2-cm mesh exposure was seen in one patient on the first-month postoperative follow-up. Despite the vaginal estrogen treatment, it could not be recovered and, the ~2-cm mesh was excised by a transvaginal approach at the seventh postoperative month. The patient was discharged and followed without any problems. In the literature, the studies conducted about VALSH/C reported a 0–10% rate of mesh-related complications [the cumulative ratio of 2.3 (13/574)] (8,17-30). Nosti et al. compared the mesh-related complications between VALSC (vaginal mesh attachment) and standard laparoscopic sacrocervicopexy (LSCx) (laparoscopic mesh attachment), and found no difference between the groups (1.6% VALSC vs. 1.7% LSCx; P=1.0) (30). The mesh-related complications rate is not higher in VALSH/C than those seen in standard LSH/C (5,16).
Our primary concern with this technique is the unconscious transport of a vaginal infection through the ascending route to the abdominal cavity and/or the retroperitoneal region, and the development of infection-related complications. Fortunately, this situation has not been seen in any of our patients. In the literature, no moderate/severe infection-related complications were reported in any of the studies, which have reported more than 550 VALSH/C patients in total (8,17-30). Rae et al. (17) reported postoperative pyrexia and urinary tract infection in four patients (18.2%). Aharoni et al. (22) and Zhu et al. (24) reported postoperative fever in 2 (4.4%) and 1 (4.8%) patients respectively, and all authors stated that the patients recovered without any problems (17,22,24). Nevertheless, we believe that it is important to follow the prophylactic methods described above in tips and tricks to prevent infection. If the patient has any signs or suspicion of a pelvic infection, surgery should be postponed until full recovery. Besides, as a precaution, we give prophylactic antibiotherapy for 5 days after surgery, but the usefulness of this is subject to debate.
In our technique, the mesh is anchored to the anterior longitudinal ligament of the sacral promontory using a titanium tacker. This implementation is a controversial issue. Most urogynecologists avoid using titanium tacks in the anterior longitudinal ligament, as they might cause postoperative sacral osteitis/discitis. In the literature, nine researchers used a tacker for mesh fixation in their VALSH/C case series (8,17-20,22,23,26,29), and none of them reported any osteitis/discitis complications (over 350 cases in total). Only, Godin et al. reported an extremely different complication related to the helical coil (Origin Tacker System) in one patient, defined as second sacral neural root injury that required revision laparotomy (18). In their series, an average of seven coils was used for fixation, and in our opinion, the tacker might have unintentionally slid too much to the left due to the use of so many coils. In our technique, a maximum of three coils are applied and gently fixed, and no such complication was observed. The tacker use seems to shorten the operation time and opens the door for more gynecologic endoscopists. Moreover, eliminating the suturing step on the sacral promontory can prevent any presacral vessel injuries that might occur during the process. As a result, we believe that the tacker can be used efficiently during the anchoring process. However, the larger series with long follow-up periods are needed to speak more confidently about the subject.
In the SH/C procedure, the proper adjustment of the mesh tension is crucial for long-term symptoms and complications. The tension of the mesh should be balanced very carefully, and it should be remembered that when the mesh is stretched beyond measure, complications (e.g., chronic pelvic pain, dyspareunia, and dysmenorrhea) might occur postoperatively; yet when the mesh is applied relaxed, POP recurrence might occur postoperatively. In our retroperitoneal tunneling method, the peritoneum is not opened, and the mesh is transferred via a retroperitoneal route and placed following the curve of the pelvic cavity. In this way, the tension can be adjusted effectively since the natural anatomy is not disrupted. Due to this advantage, the retroperitoneal tunneling technique is better than other VALSH/C procedures in which the peritoneum is opened as a whole. Besides, the procedure without opening the peritoneum provides the elimination of the suturing step and significantly reduces the operation time (8,20,23).
The conversion rate to either laparotomy or vaginal approach rate for standard LSH/C due to strategic and/or complication reasons was reported as 0–11% (16). In a randomized controlled trial (RCT), Lucot et al. have reported a conversion rate of 6.3% for standard LSH/C because of strategic reasons, such as the inability to access the promontory and adhesions (31). In another recently published RCT (LAVA trial), the authors reported a relatively high rate of conversion from laparoscopy to vaginal approach (6/64, 9.4%) (32,33). The significant reasons for conversion are intraabdominal adhesions, excessive intraabdominal fat tissue, enlarged uterus, the inability to access the promontory, and failure in suturing and perforation of the vaginal wall during dissection. We believe that surgeons can easily overcome these difficulties by using the VALSH/C with retroperitoneal tunneling technique. In parallel with our opinion, no conversion to laparotomy or vaginal approach has been reported in studies related to VALSH/C (0/574) (8,17-30). However, the conversion risks should always be taken into consideration, and each laparoscopic surgeon, planning to perform standard or vaginal-assisted LSH/C, should also be competent to perform, at least, one alternative technique of vaginal reconstructive surgery.
Pacquée et al. performed a prospective cohort study on 331 consecutive patients who underwent standard LSC. These authors found that the anatomical failure rate at point-C was 8.6%, and that anterior (22.2%) and posterior (28%) prolapse were more common than apical prolapse at a median follow-up of 85.5 months (34). In a prospective observational study, three women (2%) required reoperation for apical support at a mean follow-up of 2.1 years (35). Thomas et al. reported recurrent stage 2 or more POP in 13.8% patients after 0.5 median years (2 days to 13.4 years) from LSC (36). In a recent RCT trial, the surgical failure rate was 1.6% for LSC 12 months postoperatively (32). Correlated with these ratios, the rates of recurrent POP requiring reoperation were 0–31% in VALSH/C studies (total 5.9% recurrence rate at an average of 23.7 months follow-up) (8,17-30). In our patients, there was no > Stage 2 POP recurrence requiring surgery. With these findings and evidence, the VALSH/C procedure appears to be an applicable, easy and effective method for apical prolapse patients.
Another critical point is the high incidence of anterior and posterior recurrence after standard LSH/C in multicompartment prolapse (33,37). In moderate-severe cystocele concomitant cases, the LSH/C procedure should be adapted to ensure that the anterior mesh is attached as low and close as possible to the bladder neck. This concept is also the same for the posterior compartment prolapse. Nevertheless, the deep caudal dissection of the anterior and posterior vaginal wall, which is often required for optimal mesh placement, is sometimes technically challenging. The manipulation and suturing in the deep pelvis is not easy; sometimes, the mesh is anchored to a more proximal level, and therefore, suboptimal placement of the mesh might occur. In the VALSH/C technique, the process starts from the vaginal part, and that allows us a multicompartment revision, including anterior and posterior compartments and enterocele repairing. We always perform a concomitant procedure for stage 3 or higher anterior and posterior prolapse and enterocele cases via the vaginal route. This approach can explain the absence of recurrence requiring surgery in our patients.
There are few published studies addressing the VALSH/C procedure. Among these, nine of them presented their reports of VALSC (17,19,22,24,25,27-30), one presented both VALSC and VALSH (18), and the remaining five introduced VALSH cases (8,20,21,23,26). All of these studies concluded that the technique is feasible, safe, and effective. Three of 15 transferred the mesh to promontory via a retroperitoneal tunneling route [two used an ascending route (8,23), and the other used a descending route (20)], the others put the mesh into the peritoneal cavity and, then continued their technique in a conventional style (17-19,21,22,24-30). Only, Sanverdi et al. and our team have performed the ascending retroperitoneal transfer approach (8,23). It facilitates the technique, and VALSH/C is becoming more applicable with eliminating the peritoneal suturing. Also, in this way, the mesh is placed more anatomically and naturally. Sanverdi et al. anchored the mesh only onto the posterior lip of the cervix, distinct from our technique (23). We anchored the mesh onto both the anterior and posterior lips of the cervix (8), and we believe that this approach carries the uterus more anatomically compare to posterior mesh only technique. In Table 1, the VALSH/C procedures and their outcomes are presented in detail (Table 1).
Table 1
| Author [year] | Study type | Procedure/patients [n] | Operation time (min) [min–max] | EBL (mL) [min–max] | Hospital stay (day) [min–max] | Follow-up period (month) [min–max] | Complications/outcomes |
|---|---|---|---|---|---|---|---|
| Godin et al. [1999] (18) | Case series | Total [45]; VALSH [5], VALSCx [22],VALSC [18] | 73.9±17.5, 63±6.7, 116.5±15, 84.4±23.4 | No remarkable bleeding | 4±1, 4±1, 4±1, 4±1 | 13 [6–30] | VALSC group: second left sacral neural root injury, 1 (revision LPT was performed); recurrent enterocele, 1 |
| VALSCx group: recurrent apical prolapse, 1 | |||||||
| Rae et al. [2002] (17) | Case series | VALSC [22] | 105 [70–180] | N/A | 3.18 [2–6] | 12.5 [3–38] | Rectal injury, 1; postop. pyrexia, 2; urinary tract infection, 2; blood transfusion, 1; mesh erosion, 2 (9.2%); recurrent cystocele, 7 (4 require treatment) |
| von Pechmann et al. [2011] (27) | Comparative | VALSC [44] vs. LSC [26] | 215.2±41 vs. 269.7±55.6 | N/A | 2 [1–11] vs. 2 [1–9] | 125 [20–611] vs. 223 [36–743] days | Intraoperative: serosal small bowel injury, 1 vs. 0; cystotomy, 0 vs. 1 |
| Postoperative: ileus, 1 vs. 0; acute renal failure caused by ketorolac, 1 vs. 0; abdominal cellulitis, 1 vs. 0; postoperative SUI, 7 vs. 3; mesh extrusion, 1 (2.3%) vs. 0 | |||||||
| Athanasiou et al. [2013] (19) | Case series | VALSC [27] | 74 [60–120] | 310 [250–400] | 2.8 [2–5] | 12 | De novo constipation, 3; prolene suture visibility at vaginal vault, 1 |
| Zhu et al. [2013] (24) | Case series | VALSC [21] | 95.6 [85–150] | 147 (N/A) | N/A | 43.5 [18–60] | Postoperative fever, 1; mesh exposure, 1 (4.8%); postoperative SUI (6th months), 1 |
| Fayyad et al. [2014] (26) | Case series | VALSH [70] | 122 [45–150] | 100 [50–200] | 1.5 [22 h–3 days] | 12 | Bladder injury, 2; pelvic hematoma, 2; de novo SUI, 6; mesh comp., 2 (2.9%); recurrent uterine prolapse, 3; recurrent anterior vaginal prolapse, 10 (total 6 required reoperation) |
| Elvira et al. [2014] (21) | Comparative | VALSH [18] vs. LSH [14] | 102.8±20.1 [77–138] vs. 93.24±22.2 [60–144] | 115±18.4 vs. 120±20.6 | 2 vs. 2 | 34 [12–70] | Recurrent 2° cystocele, 0 vs. 3 |
| Liang et al. [2016] (25) | Case series | VALSC [30] | 105.3±25.8 | 93.8±45.2 | 4 [2–12] | 36 | Pelvic hematoma (grade III), 2; urinary retention, 1 (resolved); lumbosacral pain, 1 (postoperative 3th years); de novo SUI, 2 (postoperative 3th years); de novo constipation, 4 (postoperative 3th years); de novo dyspareunia, 2 (postoperative 3th years); mesh comp., 3 (10%) (2 exposure, 1 erosion) |
| Grigoriadis et al. [2015] (28) | Case report | VALSC [1] | N/A | N/A | N/A | N/A | N/A |
| Nosti et al. [2016] (30) | Comparative | VALSC [123] vs. LSCx [59] | 256±53 vs. 344±81 | 144±95 vs. 115±82 | N/A | 21 [9–31] vs. 24 [9–39] | Mesh comp., 1.6% (2/123) vs. 1.7% (1/59); suture erosion, 1% vs. 2%; anatomic success, 94.4% vs. 93.2% |
| Darwish et al. [2018] (20) | Case series | VALSH-dRT [15] | VP: 8.54±3.1 [7–12], LP: 32.36±8.2 [27–41]; Total: 30–50 | <1 g/dL reduction in Hb, 2 | 2.8 | 6 | Recurrent 2° POP, 1 (6.7%) |
| Aharoni et al. [2017] (22) | Comparative | VALSC [45] vs. LSC [28] | 84 [54–122] vs. 92 [N/A] | N/A | 2 [N/A] vs. 2 [N/A] | [12–60] vs. [36–84] | Postoperative fever, 2 vs. 1; transient urinary retention, 1 vs. 0; recurrent uterine prolapse at postoperative 3 weeks, 0 vs. 1; subjective cure rate at 1–5 years after the operation, 88% vs. 73% |
| Sanverdi et al. [2017] (23) | Case series | VALSH-aRT [33] | 59.5 [20–120] | No remarkable bleeding | N/A | 12 | No recurrence and/or mesh complication were occurred |
| Tapisiz et al. [2018] (8) | Case report | VALSH-aRT [1] | 90 | 200 | 2 | 6 | No recurrence and/or mesh complication were occurred |
| Athanasiou et al. [2019] (29) | Case series | VALSC [94] | N/A | N/A | N/A | 84 [36–120] | Mesh extrusion, 2 (2.1%); anatomic recurrence, 3 (2 required reoperation) |
| Tapisiz et al. [2020] (Our series’ unpublished data) | Case series | Total [21]; VALSH-aRT [18], VALSC-aRT [2], VALSH + MF-aRT [1] | 70 [60–100] | <100 | 2 [2–6] | 20 [5–52] | Pelvic pain, 3 (9.6%) (resolved in postoperative 3 months); mesh exposure, 1 (4.8%) (revision operation was performed by a TV approach); recurrent 2° cystocele, 2 (no requiring surgery) |
VALSC, vaginally-assisted laparoscopic sacrocolpopexy; VALSH, vaginally-assisted laparoscopic sacrohysteropexy; LSC, laparoscopic sacrocolpopexy; LSCx, laparoscopic sacrocervicopexy; aRT, ascending retroperitoneal tunneling; dRT, descending retroperitoneal tunneling; SUI, stress urinary incontinence; VP, vaginal phase; LP, laparoscopic phase; Hb, hemoglobin; comp., complication; MF, Manchester-Fothergill operation; TV, transvaginal.
To our knowledge, this is the first literature review addressing the VALSH/C procedure. Our review is also supplemented with critical tips and tricks about the procedure, which will help to achieve standardization. However, we are aware of the possibility of missed articles, as we limited the search results to articles written in English only, which were published from 1994 onwards. On the other hand, the main limitation of this study is that the results of our series cannot be presented in detail as they are under preparation.
Conclusions
VALSH/C appears to be a feasible, safe, simple, effective, and valid option for POP. The combination of the vaginal and laparoscopic approaches is useful in obtaining a variant of sacropexy, which is as minimally invasive as possible and has a short operative time. The vaginal approach provides a safe and sufficient condition for suturing the mesh to the cervix or vaginal wall and repairing concurrent vaginal wall prolapse. Besides, it seems that retroperitoneal tunneling makes the process even more comfortable. Further studies are needed to compare these techniques with other pelvic reconstructive procedures. VALSH/C is a multicompartment minimally invasive, feasible, old-new solution for POP, like “old wine in a new bottle”.
Acknowledgments
We would like to thank to guest editor Dr. Gokhan Sami Kilic for kind invitation and support.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Gokhan Kilic) for the series “Minimally Invasive Treatment Modalities for Female Pelvic Floor Disorders” published in Gynecology and Pelvic Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm-2020-pfd-06/coif). The series “Minimally Invasive Treatment Modalities for Female Pelvic Floor Disorders” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All these figures are from our own operations and all of them are original. The patients signed an informed consent that allows us to use their data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010;29:4-20. [Crossref] [PubMed]
- Anger JT, Mueller ER, Tarnay C, et al. Robotic compared with laparoscopic sacrocolpopexy: A randomized controlled trial. Obstet Gynecol 2014;123:5-12. [Crossref] [PubMed]
- Maher C, Feiner B, Baessler K, et al. Surgery for women with apical vaginal prolapse. Cochrane Database Syst Rev 2016;10:CD012376. [Crossref] [PubMed]
- Heft JS, Adam RA. Apical Prolapse: Is There a Best Approach?. Curr Bladder Dysfunct Rep 2018;13:101-10. [Crossref]
- Schachar JS, Matthews CA. Updates in Minimally Invasive Approaches to Apical Pelvic Organ Prolapse Repair. Curr Obstet Gynecol Rep 2019;8:26-34. [Crossref]
- Tapısız ÖL, Dogan AR, Kiykac Altinbas S. Is vaginally assisted laparoscopic sacrohystero/colpopexy a feasible technique? Turk J Med Sci 2018;48:1372-4. [Crossref] [PubMed]
- Tapisiz OL, Dogan AR, Kiykac Altinbas S, et al. Vaginal assisted laparoscopic sacrohysterocervicopexy: Video presentation, 14th National Turkish Society of Obstetrics and Gynecology Congress, “The Best Video Presentation Award”, October 05-09 2016, Antalya, Turkey. Available online: https://www.tjod.org/tjod-2016-odulleri/ [accessed 31 May 2020].
- Tapisiz OL, Dogan AR, Kiykac Altinbas S. Vaginal-assisted laparoscopic sacrohysterocervicopexy with retroperitoneal tunneling. Int J Gynaecol Obstet 2018;140:118-9. [Crossref] [PubMed]
- Siddiqui NY, Grimes CL, Casiano ER, et al. Mesh sacrocolpopexy compared with native tissue vaginal repair: a systematic review and meta-analysis. Obstet Gynecol 2015;125:44-55. [Crossref] [PubMed]
- Linder BJ, Occhino JA, Habermann EB, et al. A national contemporary analysis of perioperative outcomes of open versus minimally invasive sacrocolpopexy. J Urol 2018;200:862-7. [Crossref] [PubMed]
- Mowat A, Maher C, Pelecanos A. Can the learning curve of laparoscopic sacrocolpopexy be reduced by a structured training program? Female Pelvic Med Reconstr Surg 2018;24:272-6. [Crossref] [PubMed]
- Claerhout F, Roovers JP, Lewi P, et al. Implementation of laparoscopic sacrocolpopexy--a single centre's experience. Int Urogynecol J Pelvic Floor Dysfunct 2009;20:1119-25. [Crossref] [PubMed]
- Mustafa S, Amit A, Filmar S, et al. Implementation of laparoscopic sacrocolpopexy: establishment of a learning curve and short-term outcomes. Arch Gynecol Obstet 2012;286:983-8. [Crossref] [PubMed]
- Akladios CY, Dautun D, Saussine C, et al. Laparoscopic sacrocolpopexy for female genital organ prolapse: establishment of a learning curve. Eur J Obstet Gynecol Reprod Biol 2010;149:218-21. [Crossref] [PubMed]
- Kantartzis K, Sutkin G, Winger D, et al. Introduction of laparoscopic sacral colpopexy to a fellowship training program. Int Urogynecol J. 2013;24:1877-81. [Crossref] [PubMed]
- Ganatra AM, Rozet F, Sanchez-Salas R, et al. The current status of laparoscopic sacrocolpopexy: a review. Eur Urol 2009;55:1089-103. [Crossref] [PubMed]
- Rae D, Hawthor R. Sacrocolpopexy for vaginal vault prolapse: a combined vaginal and laparoscopic approach. Gynaecological Endoscopy 2002;11:75-7. [Crossref]
- Godin PA, Nisolle M, Smets M, et al. Combined vaginal and laparoscopic sacro®xation for genital prolapse using a tacking technique: a series of 45 cases. Gynaecological Endoscopy 1999;8:277-85. [Crossref]
- Athanasiou S, Grigoriadis T, Chatzipapas I, et al. The vaginally assisted laparoscopic sacrocolpopexy: a pilot study. Int Urogynecol J 2013;24:839-45. [Crossref] [PubMed]
- Darwish A, Bahlol M, Ahmad A, et al. Uterus-sparing vaginolaparoscopic sacrocolpopexy for apical pelvic organ prolapse. Int Urogynecol J 2018;29:1455-61. [Crossref] [PubMed]
- Elvira BV, Brătilă PC, Negroiu AT. Vaginally-Assisted Laparoscopic Hysterosacropexy for Advanced Utero-Vaginal Prolapse: A Series of 32 Cases. ARS Medica Tomitana 2014;20:63-70. [Crossref]
- Aharoni A, Mamet Y, Agranat A. Efficacy of vaginal and laparoscopic sacrocolpopexy (VLSCP), a dual approach to utero-vaginal prolapse, compared with laparoscopic sacrocolpopexy (LSCP) alone. Pelviperineology 2017;36:113-4.
- Sanverdi İ, Kilicci C, Polat M, et al. A new operation technique for uterine prolapse: Vaginally-assisted laparoscopic sacrohysteropexy. Turk J Obstet Gynecol 2017;14:181-6. [Crossref] [PubMed]
- Zhu L, Sun Z, Yu M, et al. Modified laparoscopic sacrocolpopexy with mesh for severe pelvic organ prolapse. Int J Gynaecol Obstet 2013;121:170-2. [Crossref] [PubMed]
- Liang S, Zhu L, Song X, et al. Long-term outcomes of modified laparoscopic sacrocolpopexy for advanced pelvic organ prolapse: A 3-year prospective study. Menopause 2016;23:765-70. [Crossref] [PubMed]
- Fayyad AM, Siozos CS. Safety and one year outcomes following vaginally assisted laparoscopic uterine sacropexy (VALUES) for advanced uterine prolapse. Neurourol Urodyn 2014;33:345-9. [Crossref] [PubMed]
- von Pechmann WS, Aungst MJ, Gruber DD, et al. A pilot study on vaginally assisted laparoscopic sacrocolpopexy for patients with uterovaginal prolapse. Female Pelvic Med Reconstr Surg 2011;17:115-9. [Crossref] [PubMed]
- Grigoriadis T, Protopapas A, Chatzipapas I, et al. Vaginally assisted laparoscopic sacrocolpopexy for the treatment of complete uterovaginal prolapse. Int Urogynecol J 2015;26:449-50. [Crossref] [PubMed]
- Athanasiou S, Zacharakis D, Protopapas A, et al. Severe pelvic organ prolapse. Is there a long-term cure? Int Urogynecol J 2019;30:1697-703. [Crossref] [PubMed]
- Nosti PA, Carter CM, Sokol AI, et al. Transvaginal versus transabdominal placement of synthetic mesh at time of cacrocolpopexy. Female Pelvic Med Reconstr Surg 2016;22:151-5. [Crossref] [PubMed]
- Lucot JP, Cosson M, Bader G, et al. Safety of vaginal mesh surgery versus laparoscopic mesh sacropexy for cystocele repair: Results of the prosthetic pelvic floor repair randomized controlled trial. Eur Urol 2018;74:167-76. [Crossref] [PubMed]
- van IJsselmuiden MN, van Oudheusden A, Veen J, et al. Hysteropexy in the treatment of uterine prolapse stage 2 or higher: laparoscopic sacrohysteropexy versus sacrospinous hysteropexy-a multicentre randomised controlled trial (LAVA trial). BJOG 2020;127:1284-93. [Crossref] [PubMed]
- de Tayrac R. Can laparoscopic sacrohysteropexy treat all pelvic organ prolapses? BJOG 2020;127:1294. [Crossref] [PubMed]
- Pacquée S, Nawapun K, Claerhout F, et al. Long-term assessment of a prospective cohort of patients undergoing laparoscopic sacrocolpopexy. Obstet Gynecol 2019;134:323-32. [Crossref] [PubMed]
- Rahmanou P, White B, Price N, et al. Laparoscopic hysteropexy: 1- to 4-year follow-up of women postoperatively. Int Urogynecol J 2014;25:131-8. [Crossref] [PubMed]
- Thomas TN, Davidson ERW, Lampert EJ, et al. Long-term pelvic organ prolapse recurrence and mesh exposure following sacrocolpopexy. Int Urogynecol J 2020;31:1763-70. [Crossref] [PubMed]
- Chang OH, Davidson ERW, Thomas TN, et al. Does concurrent posterior repair for an asymptomatic rectocele reduce the risk of surgical failure in patients undergoing sacrocolpopexy? Int Urogynecol J 2020;31:2075-80. [Crossref] [PubMed]
Cite this article as: Tapisiz OL, Kiykac Altinbas S, Dogan AR. Vaginal-assisted laparoscopic sacrohystero/colpopexy with retroperitoneal tunneling: tips and tricks, and a review of the literature. Gynecol Pelvic Med 2020;3:38.