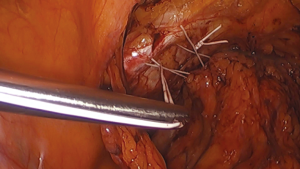
Burch colposuspension using minimally invasive techniques
Introduction
Urinary incontinence affects 4.8% to 58.4% of community dwelling women of all ages (1-4). It is one of the most prevalent chronic diseases facing the medical community. Stress urinary incontinence has a reported prevalence of 25% to 35% in the United States (5-7). Surgical management remains a popular and primary approach to treating stress incontinence. The modern operative techniques include the no-tension sling and the Burch retropubic urethropexy. Both outpatient procedures are minimally invasive—the sling via a vaginal approach and the Burch procedure via a laparoscopic or robotic approach. Both are performed with relatively short operative times (in our practice, the sling averages 15 minutes; the Burch averages 30–60 minutes). Peer-reviewed literature has demonstrated equivalent short- and long-term success rates with a low risk of complications (8,9). Due to its significantly shorter operative time and simplicity in technique, the no-tension sling has been favored over the Burch urethropexy for years. However, recent controversies surrounding vaginal mesh have led to a resurgence in interest in the Burch urethropexy as a primary incontinence operation in place of the mesh sling. This article will review the background of the Burch urethropexy and highlight some pearls about the surgery.
Background on technique development
Dr. John Burch described his technique in 1961 as a modification of the Marshall-Marchetti-Krantz urethropexy (10). He changed the suture fixation point from the ostium of the pubis to the iliopectineal line (also known as Cooper’s ligament) as a secure fixation point for sutures placed in the visceral connective tissue adjacent to the bladder neck and urethra. Dr. Emil A. Tanagho published his modifications to the procedure in 1978. He emphasized a full-thickness pass of the suture into the visceral connective tissue as far lateral from the urethra as possible. This was, in part, to minimize injury to the neurovascular tissue immediately adjacent the urethra (11). Current methodology utilizes four sutures in total. Sutures are placed bilateral to the urethrovesical junction and bilateral to the proximal urethra (Figure 1). When tied down, a suture bridge between the visceral connective tissue and the iliopectineal line is created to mitigate urethral hypermobility. Overcorrection is avoided to reduce the risk of potential voiding dysfunction. Adhering to these principles have shown high success rates, although the exact mechanism of how this procedure promotes continence remains poorly understood.
Preoperative incontinence assessment
Patients with a history of stress urinary incontinence should undergo a pelvic exam documenting pelvic support, assessment of urethral hypermobility, and clinically demonstrate stress urinary incontinence. The patient should have no complaints of voiding dysfunction and a normal post-void residual should be documented.
Those with demonstrable stress incontinence and urethral hypermobility as well as a clinical absence of intrinsic sphincter deficiency and no evidence of voiding dysfunction are considered good surgical candidates.
Patients with a fixed urethra, suspected or confirmed intrinsic sphincter deficiency, or voiding dysfunction should be evaluated further. Alternative treatments should be considered.
Other factors that can impact success include increasing age, previous incontinence surgery, the presence of detrusor instability, and the postoperative onset of irritative bladder symptoms (12).
Anesthesia and positioning
The procedure is performed under general anesthesia. The patient is placed in a lithotomy position with Allen stirrups. A 3-lumen Foley catheter is placed in the bladder. One lumen is attached to a Foley bag. The other is attached to an infusion line of irrigation solution, allowing for retrograde filling of the bladder during the procedure. During surgery, the patient is maintained in a Trendelenburg position.
Relevant anatomy
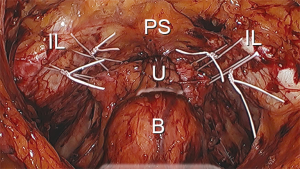
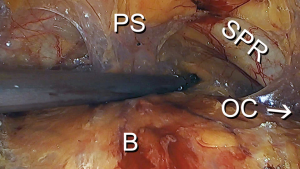
Upon entry into the space of Retzius, numerous important anatomical structures are encountered. It is important to be aware of their location and relevance to the procedure. In the midline, principal genitourinary structures including the urethra, bladder neck, and bladder are encountered. Underlying and lateral to the urethra and the bladder is the anterior visceral connective tissue of the vagina. Anteriorly and lateral to the urethra are the pubic symphysis and the superior pubic ramus, respectively. The iliopectineal line (also known as Cooper’s ligament) is located along the superior pubic ramus. The lateral pelvic sidewalls contain the obturator internus muscle, which is generally covered with fascia. The iliococcygeus muscle is typically not visible unless a paravaginal defect is present. The iliococcygeus muscle is attached to the obturator internus muscle. The fascia of the iliococcygeus muscle fuses with the fascia of the obturator internus muscle, creating a fusion called the arcus tendinous levator ani. The vagina is anchored laterally to the fascia of the obturator internus muscle, creating a fusion referred to as the arcus tendinous fascia pelvis. The “White Line” is a visible result of the fusion of the fascial planes, extending from the lateral pubic tubercle to the ischial spine (Figure 2).

The surgeon should be acutely aware of the vasculature within the space of Retzius. Lateral to the superior pubic ramus, the external iliac vein can be encountered (note: dissection should be avoided in area). The obturator artery and nerve emerge posteriorly in the space of Retzius and enter the obturator canal neighboring the superior pubic ramus. It is not unusual to find an aberrant artery or vein emanating from the external iliac artery or vein and crossing the superior pubic ramus as it enters into the obturator canal. The pelvic sidewall is otherwise relatively devoid of significant vasculature. The plexus of Santorini can be found along the anterior visceral connective tissue of the vagina and, when lacerated, can result in significant blood loss. Vasculature overlying the bladder consisting principally of venous drainage is also present (Figure 3).

The obturator nerve, which emanates from L2, L3 enters into the space of Retzius posteriorly and passes through the obturator canal alongside the obturator artery and vein.
Operative technique
Four laparoscopic ports are placed, including a 5-mm umbilical port for the laparoscope. An 8-mm port is placed on either side of the midline, just medial to the anterior superior iliac crest. A final 5-mm port is placed above the symphysis pubis and bladder reflection.
The Foley catheter bag tubing draining the bladder is clamped and the bladder is filled in retrograde fashion with approximately 200 to 300 cc of irrigation solution. This aids in delineating the margins of the bladder. An incision is made several centimeters superior to the bladder reflection utilizing an ultrasonic scalpel. The incision is carried out laterally to the medial margin of the obliterated umbilical ligament on either side of the midline. As the space of Retzius is entered, the distended bladder aids dissection as gravity pulls it away from the anterior abdominal wall. Once adequate dissection has been achieved, the bladder is drained. Blunt dissection is performed in the space of Retzius, separating the bladder off the anterior abdominal wall—principally in the midline. Once the pubic tubercle is identified, sidewall structures are then located. This includes the superior pubic ramus, the iliopectineal line, the obturator internus muscle with its overlying fascia, the White Line, neighboring visceral connective tissue, and the obturator canal with its associated neurovascular plexus. Dissection is performed in the midline to establish landmarks before carefully moving laterally to allow for early identification of the obturator canal and reduce risk of injury to the neurovascular plexus (Figure 4).
Fatty tissue overlying the pelvic sidewall is frequently encountered. It can be left in place or teased away beginning in the midline and working laterally if needed, to identify the White Line, obturator canal, or other sidewall structures with better clarity. There is little fear of bleeding due to the avascular nature of the pelvic sidewall when remote from the neurovascular plexus. As well, adipose tissue overlying the anterior visual connective tissue lateral to the urethra and bladder can also be gently grasped and pulled free with minimal risk of bleeding (Figure 5). Mobilization of fatty tissue overlying the bladder should be limited or avoided due to risk of significant adherence of the fatty tissue to the venous plexus overlying the bladder, which could cause bleeding. Fatty tissue overlying the urethra and bladder neck should be minimized or avoided to reduce risk of injury to the neurovascular bundle overlying the structures.
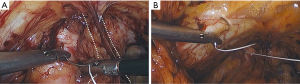
Permanent suture, such as 2-0 polytetrafluoroethylene, is loaded onto a laparoscopic needle driver. The bladder neck is identified by visualizing the location of the Foley bulb. To aid in suture placement, a finger can elevate the anterior vaginal wall adjacent to the urethra, stabilizing the tissue during need passage (Figure 6A,B). The first polytetrafluoroethylene (PTFE) suture is placed lateral to the urethra adjacent to the pubic tubercle (Figure 7A). The suture can be doubly passed with the purchases to the anterior fascial connective tissue. It is then passed through the iliopectineal line but not tied down (Figure 7B). The second suture is placed lateral to the bladder neck and secured to a neighboring portion of the iliopectineal line (Figure 8A,B). Sutures are then tied down. The bladder neck is elevated by the assistant’s finger in the vagina to establish an orientation parallel to the arcus tendinous fascia pelvis. A suture bridge is created to avoid overcorrection which could lead to voiding dysfunction (Figure 9). The procedure is repeated on the opposite side (Figure 1).
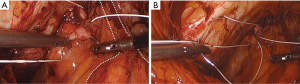
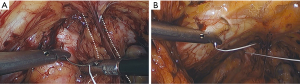
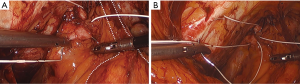
The peritoneal incision created to access the space of Retzius is closed in a transverse fashion with a delayed absorbable barbed suture.
Cystoscopy is performed at the completion of the procedure. The bladder, bladder neck, and urethra are examined for evidence of injury or other abnormalities including the presence of a Burch urethropexy suture within the urethra or bladder. The ureteral orifices are observed for evidence of efflux to confirm ureteral patency. Efflux of urine should be vigorous to help verify an absence of partial obstruction of the ureter. Once cystoscopy is completed, the bladder is drained with a Foley catheter.
Postoperative management
A voiding trial can be performed once the patient is awake and ambulatory. The patient’s bladder can be retrograde filled through the Foley catheter with approximately 250 cc of irrigation solution. The Foley catheter is removed and the patient is given 15 minutes to void. A post-void residual is measured either by catheter or bladder ultrasound. A residual of ≤100 cc is deemed adequate for the Foley catheter to be left out. However, if the residual is >100 cc, the Foley catheter will be replaced and a voiding trial is again attempted in 4–7 days.
Comments (tips and tricks)
- The incision to enter the space of Retzius should be several centimeters cephalad to the distended bladder along the anterior abdominal wall. Avoid dissecting lateral to the obliterated umbilical artery, where the inferior epigastric vessels are located and risk laceration. During entry into the space of Retzius, favor the dissection toward the rectus abdominis muscles and away from the bladder to reduce risk of an incidental cystotomy;
- Hydrodissection with a laparoscopic irrigator following the initial incision to access the space of Retzius aids in identifying planes of dissection;
- Do not dissect lateral to the superior pubic ramus as this region contains the iliac vein which, if injured, can bleed profusely;
- When removing fat from the sidewall or anterior visceral connective tissue, only grasp the superficial surface of the fat and not the underlying deeper tissue. Grasping deeper tissue could potentially cause bleeding;
- Avoid dissection directly on the urethra or bladder neck to minimize risk of injury to the neurovascular plexus of either structure;
- If concerned of an incidental needlestick into the finger that is elevating the anterior vaginal wall while a needle is passed through the tissue, a sterile thimble can be placed over the vaginal finger to reduce this risk;
- It is best to place the distal suture first, lateral to the proximal/middle third of the urethra. Placement of the bladder neck suture first can interfere with placement of the distal suture;
- Deep purchases of the fascia are necessary. A superficial bite may pull through and possibly cause bleeding from the underlying venous plexus;
- When sutures are placed near a vein, the sutures should be passed deep into the tissue going around the vein. If some bleeding does occur, it often resolves once the suture bridge has been tied down. However, if the bleeding is significant, the vein can be coagulated with bipolar cautery. Alternatively, the Burch suture can be tied down against the anterior visceral connective tissue to stop the bleeding prior to placement of the suture in the iliopectineal line (also known as Cooper’s ligament);
- The iliopectineal line (also known as Cooper’s ligament) is a thick fibrous structure that will readily accept passage of a needle underneath it. The needle should be angled only slightly inward but otherwise remain parallel to the ligament. This will ease passage of the needle and avoid getting stuck in the bone;
- The surgeon and assistant can each tie down separate sutures simultaneously, reducing surgical time;
- It is suggested to make sure that both sides of Burch sutures equally suspend the bladder neck and urethra. Disproportional tensioning between the two sides could potentially impact direction of the urinary stream—particularly when the patient is squatting over a toilet;
- Cystoscopy should always be performed after the surgery to ensure no sutures have been placed into the urethra or bladder;
- Caution is made when placing the cystoscope through the urethra into the bladder due to diminished mobility of the urethra and a change in orientation of the urethra as result of the Burch urethropexy;
- Voiding dysfunction immediately following the surgery can occur and can occasionally persist beyond 7 days. As a result, some physicians choose to use a suprapubic catheter, allowing the patient to determine her ability to void at home without making repeated trips to the office for a voiding trial;
- In the rare circumstance of urinary retention, this may require reoperation with revision of the sutures.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Gokhan Kilic) for the series “Minimally Invasive Treatment Modalities for Female Pelvic Floor Disorders” published in Gynecology and Pelvic Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-44/coif). The series “Minimally Invasive Treatment Modalities for Female Pelvic Floor Disorders” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Minassian VA, Drutz HP, Al-Badr A. Urinary incontinence as a worldwide problem. Int J Gynaecol Obstet 2003;82:327-38. [Crossref] [PubMed]
- Anger JT, Saigal CS, Litwin MS, et al. The prevalence of urinary incontinence among community dwelling adult women: results from the National Health and Nutrition Examination Survey. J Urol 2006;175:601-4. [Crossref] [PubMed]
- Hunskaar S, Lose G, Sykes D, et al. The prevalence of urinary incontinence in women in four European countries. BJU Int 2004;93:324-30. [Crossref] [PubMed]
- Melville JL, Katon W, Delaney K, et al. Urinary incontinence in US women: a population-based study. Arch Intern Med 2005;165:537-42. [Crossref] [PubMed]
- Hampel C, Artibani W, Espuña Pons M, et al. Understanding the burden of stress urinary incontinence in Europe: a qualitative review of the literature. Eur Urol 2004;46:15-27. [Crossref] [PubMed]
- Hampel C, Wienhold D, Benken N, et al. Definition of overactive bladder and epidemiology of urinary incontinence. Urology 1997;50:4-14; discussion 15-7. [Crossref] [PubMed]
- Markland AD, Richter HE, Fwu CW, et al. Prevalence and trends of urinary incontinence in adults in the United States, 2001 to 2008. J Urol 2011;186:589-93. [Crossref] [PubMed]
- Jelovsek JE, Barber MD, Karram MM, et al. Randomised trial of laparoscopic Burch colposuspension versus tension-free vaginal tape: long-term follow up. BJOG 2008;115:219-25. [Crossref] [PubMed]
- Dean N, Herbison P, Ellis G, et al. Laparoscopic colposuspension and tension-free vaginal tape: a systematic review. BJOG 2006;113:1345-53. [Crossref] [PubMed]
- Burch JC. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol 1961;81:281-90. [Crossref] [PubMed]
- Tanagho EA. Colpocystourethropexy: the way we do it. J Urol 1976;116:751-3. [Crossref] [PubMed]
- Stanton SL, Cardozo L, Williams JE, et al. Clinical and urodynamic features of failed incontinence surgery in the female. Obstet Gynecol 1978;51:515-20. [Crossref] [PubMed]
Cite this article as: Lotze PM, Mostajeran K, Li A, Lotze E, Ironside T. Burch colposuspension using minimally invasive techniques. Gynecol Pelvic Med 2020;3:37.