Robotic-assisted sacrocolpopexy steps: a narrative review
Introduction
Pelvic floor disorders such as pelvic organ prolapse (POP), urinary, and fecal incontinence are affecting more than 25% of women in the United States. The prevalence is increasing by age, and more than 10% of women in the US undergo surgical treatment for these at least once in their lifetime (1,2).
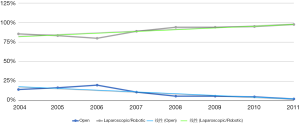
First described in 1962 by Lane, open abdominal sacrocolpopexy involves the suspension of the vagina to the sacral promontory with a graft (3). It is a safe and effective procedure that has been accepted as the gold standard for repairing apical vault prolapse. The advent of minimally invasive surgery and specifically robotically assisted laparoscopic approach has led to improved visibility, shorter surgical time, and hospital stay (4). With the approval of the da Vinci Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA, USA) by FDA for use in gynecologic surgery in 2005, the number of the sacrocolpopexy procedures increased significantly starting in 2008 (5-7) (Figure 1). Therefore, the adaptation of robotic sacrocolpopexy within the pelvic reconstructive surgery field has become the mainstay.
In this review article we present the practical steps of robotic-assisted sacrocolpopexy (RASC) in women with POP and discuss the currently available literature regarding surgical outcomes and complications of RASC.
Methods
A comprehensive review of the literature was conducted in Medline, Scopus, the Cochrane library, and Embase electronic databases using the keywords: ‘sacrocolpopexy’, ‘sacral colpopexy’, and ‘promontofixation. The approval of the da Vinci robotic surgical platform system (Intuitive Surgical; Sunnyvale, CA, USA) by the U.S. Food and Drug Administration for use in gynecologic surgery was in 2005. Therefore, we decided to conduct the current review based on articles published from that point of time to date. The date of the last search was January 26, 2020. Only full journal articles published in English since January 1, 2005, were included, with some select older references in the case of landmark papers or if secondarily referenced in studies of interest.
Procedure
Preoperative evaluation
Consideration for the surgical repair of POP is based on adequate assessment of the patient's symptoms and the degree of the prolapse. The severity of the symptoms may not correlate with the degree of prolapse.
Firstly, a full medical history of the patient should be obtained, including prolapse symptoms, presence of urinary incontinence (stress and urge), past surgical history, especially for prior abdominal and pelvic procedures. For women without a history of hysterectomy, following a thorough evaluation of any dysfunctional uterine bleeding or abnormal Papanicolaou smear, the patient should be counseled about concurrent hysterectomy/supracervical hysterectomy or uterus preserving sacrocolpopexy.
After detailed medical history, physical examination, including an abdominal and pelvic exam (supine and standing), should be performed. During abdominal examination, any incisional scars should be noted. POP-Q scoring is a helpful tool to assess each compartment of the prolapse independently (anterior, apex, and posteriorly). Prolapse should be evaluated prone and standing with appropriate Valsalva. Furthermore, stress urinary incontinence should be assessed with prolapse reduced and appropriate bladder volume (ideally in a sitting or standing position). If indicated, urodynamic testing with prolapse reduced (using a large Q-tips or pessary) could help in guiding and counseling regarding the concurrent anti-incontinence procedure. Authors feel strongly that if there is stage 3 or higher prolapse in the apex or anterior compartment, the upper urinary tract should be further evaluated using renal ultrasound and basic metabolic panel.
All patients must have an anesthesia evaluation to assess the perioperative risks and should receive appropriate perioperative antibiotics and deep venous thrombosis prophylaxis per guidelines (8). There is no need for formal bowel preparation (9). Women are counseled by their surgeons on alternative options to manage their prolapse and to discuss the risks and benefits of various prolapse repairs. Specifically for sacrocolpopexy, risk of pain, infection, bleeding, and damage to bowel, bladder, ureter, nerves, recurrence, and erosion need to be discussed (10).
Patient positioning
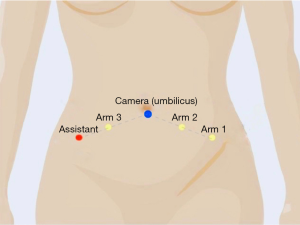
General anesthesia is administered by the anesthesiologist, and the patient is positioned securely on the operating table in the dorsal lithotomy with both arms tucked comfortably (and all bony parts padded appropriately). After the patient is prepped and draped, a Foley catheter is gently placed. Port placement may vary depending on the surgeon's preferences and characteristics of the patient. Access to the peritoneum can be obtained in an open or closed fashion (using a verses needle). Authors prefer to use all three robotic arms with an assistant port of 10–12 mm. A 12-mm umbilical trocar is placed using the open Hasson technique (11). After insufflation, three 8-mm robotic trocars and assistant port are placed bilaterally in reverse "V" configuration under direct vision (Figure 2).
Then the operating room table is lowered to the lowest level, and the patient is placed in steep Trendelenburg to allow for adequate access for the robotic arms and to provide better removal of the bowel out of the pelvis. The da Vinci robot may be positioned between the patient's legs or on the side for docking. Nowadays, with Si platform, 'side-docking' may provide a better access to the vagina for manipulation by the bedside assistant. After appropriate docking, a monopolar scissor is placed in the first robotic arm, a fenestrated bipolar grasper, and prograsp forceps are placed in the second and the third arm, respectively.
Vaginal dissection
For creating the vesicovaginal and rectovaginal spaces for mesh attachment, a sponge stick or a stainless steel end-to-end anastomosis (EEA) sizer may be used by the assistant to manipulate the vagina. ALLY Uterine Positioning System (CooperSurgical Inc., CT, USA), a table-mounted system may also be used to manipulate the vagina during the dissection to free the assistant. After localization, the peritoneum is incised at the vaginal apex with monopolar scissors (Figure 3A). Then, an anterior vaginal plane with an appropriate length (usually to the level of trigone) is created between the vagina and bladder with sharp and blunt dissection from medial to the lateral aspect (Figure 3B). The right plane is usually bloodless and spreads easily. Gentle filling of the bladder with 150 mL saline may assist in delineating the edge of the bladder, especially for patients with large floppy bladders.
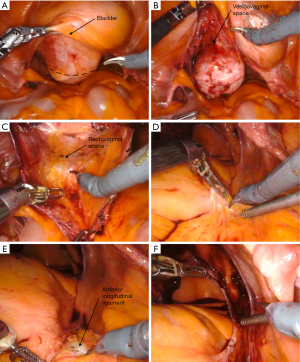
The steep Trendelenburg position may cause a challenge for posterior dissection, but upward retraction of the vagina can facilitate this step. After adjusting the vaginal manipulator to visualize the rectovaginal space, a posterior plane is created using blunt and sharp dissection and ideally is carried out distally up to the perineal body (Figure 3C). If a colpotomy is performed anteriorly or posteriorly, the injured area is closed, and the dissection is continued. If a cystotomy occurs inadvertently, the injury is closed in two layers in a watertight manner, and the patency of both ureters is verified, and the procedure is continued. In needed peritoneum covering the apex of the vagina can be used for inter-positioning.
Sacral promontory dissection
The promontory has significant landmarks around it. Before starting the dissection, for a better visualization, the right colon needs to be reflected to the left upper quadrant with the third robotic arm using the prograsp forceps. This would not only remove the bowel from the pelvis but straighten the peritoneum overlying the sacral promontory. The promontory can be identified following the pelvic rim, just below the bifurcation of common iliac arteries. The left common iliac vein is more medially than the right common iliac vessels and can be injured during exposure of the promontory (12). The right ureter is a helpful landmark on the lateral site of the promontory. Special attention is needed to identify the left common iliac vein and right ureter before sacral dissection to avoid any injury.
The peritoneum overlying promontory is lifted with the robotic grasper and is incised with the monopolar scissors (Figure 3D). Authors recommend a long enough peritoneal incision (2–3 cm) to allow for better visualization and control of bleeding if it occurs. The dissection is carried out posteriorly until the glistening white appearance of the anterior longitudinal ligament is identified (Figure 3E). Attention should be paid to the sacral vessels, and prominent veins should be cauterized. The middle sacral artery should be avoided if possible, or it can be ligated if it is directly on the suturing path.
Once the ligament is adequately exposed, there are currently two main approaches to creating a groove for attachment of the proximal part of the mesh; tunneling and non-tunneling. In tunneling technique, a tunnel under the peritoneum is created with blunt dissection from the sacral promontory distally to the level of the vaginal apex (cut surface of the posterior peritoneal dissection) without incising the peritoneum. Alternatively, in the non-tunneling technique, the peritoneal opening over promontory is incised caudally towards the vaginal cuff (Figure 3F). In both approaches, meticulous care needs to be taken to keep the right ureter away from the dissection area. The tunneling technique not only allows for faster retroperitonealization of the mesh but also provides a more natural curvature for mesh placement and avoids the risk of ureteral kinking, however, it is usually achieved in thinner patients.
Attachment of the mesh
The selection of the mesh is at the preference of the surgeon. Different types of materials can be used in sacrocolpopexy, including allografts, xenografts, autografts, or synthetic meshes (13). A type 1 macroporous polypropylene mesh has been proposed as the most appropriate material for sacrocolpopexy. The main characteristics of the mesh should be wide-pored (>75 µm) monofilament propylene Y-shaped mesh. Table 1 shows the common mesh types used for pelvic reconstruction, based on Amid’s classification system (13,14).
Table 1
| Type | Pore size | Component | Product |
|---|---|---|---|
| I | Macroporous (pore size >75 μm) | Polypropylene | Marlex |
| Atrium | |||
| Prolene | |||
| Gynemesh | |||
| Polypropylene/Polyglactin 910 | Vypro | ||
| Polyglactin 910 | Vicryl | ||
| II | Microporous | Expended polytetrafluoroethylene (PTFE) | Gore-Tex |
| III | Macroporous and microporous | Polyethylene terephthalate | Mersilene |
| PTFE | Teflon | ||
| IV | Submicronic | Not used in pelvic reconstruction |
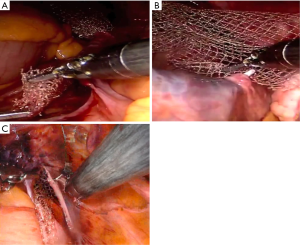
The mesh can be tailored extracorporeally according to the prepared vaginal dissection measures. Further trimming and adjustments can be done intracorporeally if needed. The authors use an already prepared Y configuration and trim it to the desired measurements (Figure 4). After placing in the abdomen, the bifurcated arms of the mesh are placed anteriorly in the vesicovaginal space and posteriorly in the rectovaginal space. Both arms of the mesh are approximately 2.5 cm in width and 6–8 cm in height. Mesh fixation is carried out with self-anchoring delayed-absorbable barbed sutures (3-0 V-Loc™, Covidien, Mansfield, USA), starting with the fixation of the anterior portion of the mesh. The suture is placed in running fashion, like drawing an S shape on the mesh at both sides (Figure 5). Additional interrupted sutures with 3-0 PDS may be placed to secure the mesh at the vaginal apex and the distal part anteriorly or posteriorly if needed for longer vaginas and dissections. The tail of the mesh may be folded under the bladder to provide a better exposure of posterior vaginal wall during mesh fixation. Studies have shown similar long-term outcomes with the use of absorbable sutures compared with non-absorbable sutures for mesh fixation (15).
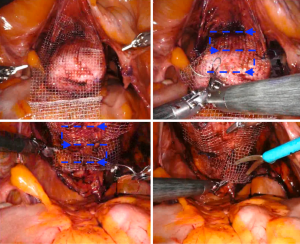
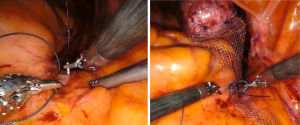
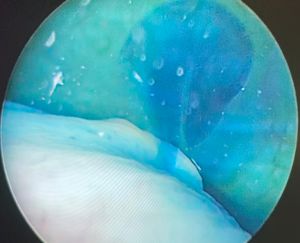
Once the fixation of the mesh to vagina is completed, the tail of the mesh is brought to the sacral promontory through the groove. Tensioning of the mesh is critical; the decision of how much pull the vaginal cuff depends on the maximal vaginal length. The procedure might be insufficient if the mesh is loose, and the patient may experience pain and discomfort if the mesh placed too tight. In our practice, this part is done manually while the surgical assistant inserts the EEA sizer into the vagina to measure the maximal vaginal length and pull back 1/3. While holding the mesh against the sacral promontory, 2 or 3 Gore-Tex® (Gore Medical, Newark, USA) or Ethibond® (Ethicon, Somerville, NJ, USA), 0 sutures are used to fixate the tail of the mesh to the anterior longitudinal ligament (Figure 6). We prefer to place these sutures vertically to avoid injuring any vessels, however they can be placed horizontally as well. Regardless, we would recommend placing these sutures broad and shallow under the ligament to provide enough strength without damaging the disk that may lay underneath the ligament. After the mesh is fully secured, redundant mesh at the promontory is removed. The mesh is then retroperitonealized using an absorbable suture (Figure 7). Intraoperative cystoscopy with indigotindisulfonate sodium (Indigo carmine™) is performed to evaluate the bladder and ureteral orifices (Figure 8).
Who would benefit from RASC
Sacrocolpopexy has superior outcomes to a variety of vaginal or abdominal procedures for apical prolapse and should be considered for patients with advanced apical prolapse who desire a highly effective procedure with better anatomical long term outcomes (16). Also, patients with recurrent prolapse who have previous failed POP surgery history, patients with young age and obesity may benefit from RASC.
Surgical outcomes
Outcomes of RASC have been reported in both objective and subjective cure rates. Objective results are based on an anatomic measurement system such as the POP-Q staging, whereas subjective results are based on patients’ reported measures using validated questionnaires. In two recent systematic reviews and meta-analysis that include 577 and 1,488 patients who underwent RASC the objective cure rate for all compartment was reported to be between 84–100%. The objective cure rate for apical prolapse was reported higher when compared to other compartments (17,18).
The definitions for subjective cure rate show heterogeneity, with some studies showing very high patient satisfaction rates changing between 90% and 100% (18-21). Culligan et al. reported a 95% cure rate in the prolapse related symptoms based in the Pelvic Floor Distress Inventory-20 (PFDI-20) questionnaire and also reported that 96% of the patients recommended sacrocolpopexy to a friend (19).
Recurrence rate was reported as 6.4% in a meta-analysis of 21 studies. The reoperation rate was reported between 2% and 26% based on long term study results, most of those being posterior colporrhaphies (10,22). Patients should be counseled about the recurrence and long term reoperation rates in the preoperative evaluation period.
Complications
Overall complication rates for robotic-assisted sacrocolpopexy are low, and many of them can be seen in both robotic and open techniques. The most common perioperative complication is mesh exposure/erosion (2–4.1%), followed by bladder injuries (2–2.8%), wound infections (2.4%), vaginotomies (1%), ureter and bowel injuries (<1%) (17,18).
The most common site of mesh exposure is the posterior vaginal wall, followed by the apex. Vaginotomy and concomitant hysterectomy is a risk factor for mesh exposure. The mesh type is also important for exposure; the lowest rate was reported with lightweight polypropylene meshes (18,23,24). Additional risks associated with the robotic procedure include port site bleeding, port site hernia, and persistent abdominal wall pain (22).
Conclusion
Robotic-assisted laparoscopic sacrocolpopexy is an efficient and safe surgical option for the repair of POP, especially for patients with anterior vault prolapse. The available data shows similar outcomes and complication rates to open technique with the advantages of minimally invasive techniques such as decreased intraoperative blood loss, decreased hospital stay, and lower overall cost.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Gokhan Kilic) for the series “Minimally Invasive Treatment Modalities for Female Pelvic Floor Disorders” published in Gynecology and Pelvic Medicine. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm-2020-pfd-05/coif). The series “Minimally Invasive Treatment Modalities for Female Pelvic Floor Disorders” was commissioned by the editorial office without any funding or sponsorship. RK is partially supported by K23DK118209, by National Institute of Heath, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wu JM, Vaughan CP, Goode PS, et al. Prevalence and Trends of Symptomatic Pelvic Floor Disorders in U.S. Women. Obstet Gynecol 2014;123:141-8. [Crossref] [PubMed]
- Fialkow MF, Newton KM, Lentz GM, et al. Lifetime risk of surgical management for pelvic organ prolapse or urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:437-40. [Crossref] [PubMed]
- Lane FE. Repair of posthysterectomy vaginal-vault prolapse. Obstet Gynecol 1962;20:72-7. [Crossref] [PubMed]
- Linder BJ, Occhino JA, Habermann EB, et al. A national contemporary analysis of perioperative outcomes for open versus minimally-invasive sacrocolpopexy. J Urol 2018;200:862-7. [Crossref] [PubMed]
- Jonsson Funk M, Edenfield AL, Pate V, et al. Trends in use of surgical mesh for pelvic organ prolapse. Am J Obstet Gynecol 2013;208:79.e1-7. [Crossref] [PubMed]
- Bouquet de Joliniere J, Librino A, Dubuisson JB, et al. Robotic Surgery in Gynecology. Front Surg 2016;3:26. [Crossref] [PubMed]
- Wang LC, Awamlh BA, Hu JC, et al. Trends in mesh use for pelvic organ prolapse repair from the Medicare database. Urology 2015;86:885-91. [Crossref] [PubMed]
- Haya N, Feiner B, Baessler K, et al. Perioperative interventions in pelvic organ prolapse surgery. Cochrane Database Syst Rev 2018;CD013105. [Crossref] [PubMed]
- Carlisle J, Swart M, Dawe EJ, Chadwick M. Factors associated with survival after resection of colorectal adenocarcinoma in 314 patients. Br J Anaesth 2012;108:430-5. [Crossref] [PubMed]
- Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA 2013;309:2016-24. [Crossref] [PubMed]
- Hasson HM. A modified instrument and method for laparoscopy. Am J Obstet Gynecol 1971;110:886-7. [Crossref] [PubMed]
- Akhgar J, Terai H, Rahmani MS, et al. Anatomical Location of the Common Iliac Veins at the Level of the Sacrum: Relationship between Perforation Risk and the Trajectory Angle of the Screw. Biomed Res Int 2016;2016.
- Chapple CR, Cruz F, Deffieux X, et al. Consensus Statement of the European Urology Association and the European Urogynaecological Association on the Use of Implanted Materials for Treating Pelvic Organ Prolapse and Stress Urinary Incontinence. Eur Urol 2017;72:424-31. [Crossref] [PubMed]
- Amid PK. Classification of biomaterials and their related complications in abdominal wall hernia surgery. Hernia 1997;1:15-21. [Crossref]
- Shepherd JP, Higdon HL, Stanford EJ, et al. Effect of suture selection on the rate of suture or mesh erosion and surgery failure in abdominal sacrocolpopexy. Female Pelvic Med Reconstr Surg 2010;16:229-33. [Crossref] [PubMed]
- Maher C, Feiner B, Baessler K, et al. Surgery for women with apical vaginal prolapse. Cochrane Database Syst Rev 2016;CD012376. [Crossref] [PubMed]
- Hudson CO, Northington GM, Lyles RH, et al. Outcomes of robotic sacrocolpopexy: A systematic review and meta-analysis. Female Pelvic Med Reconstr Surg 2014;20:252-60. [Crossref] [PubMed]
- Serati M, Bogani G, Sorice P, et al. Robot-assisted sacrocolpopexy for pelvic organ prolapse: A systematic review and meta-analysis of comparative studies. Eur Urol 2014;66:303-18. [Crossref] [PubMed]
- Culligan PJ, Gurshumov E, Lewis C, et al. Subjective and objective results 1 year after robotic sacrocolpopexy using a lightweight Y-mesh. Int Urogynecol J Pelvic Floor Dysfunct 2014;25:731-5. [Crossref] [PubMed]
- Germain A, Thibault F, Galifet M, et al. Long-term outcomes after totally robotic sacrocolpopexy for treatment of pelvic organ prolapse. Surg Endosc 2013;27:525-9. [Crossref] [PubMed]
- Chan SSC, Pang SMW, Cheung TH, et al. Laparoscopic sacrocolpopexy for the treatment of vaginal vault prolapse: with or without robotic assistance. Hong Kong Med J 2011;17:54-60. [PubMed]
- Paraiso MFR, Jelovsek JE, Frick A, et al. Laparoscopic compared with robotic sacrocolpopexy for vaginal prolapse: A randomized controlled trial. Obstet Gynecol 2011;118:1005-13. [Crossref] [PubMed]
- Belsante M, Murray S, Dillon B, et al. Mid term outcome of robotic mesh sacrocolpopexy. Can J Urol 2013;20:6656-61. [PubMed]
- Osmundsen BC, Clark A, Goldsmith C, et al. Mesh erosion in robotic sacrocolpopexy. Female Pelvic Med Reconstr Surg 2012;18:86-8. [Crossref] [PubMed]
Cite this article as: Dursun F, Khavari R. Robotic-assisted sacrocolpopexy steps: a narrative review. Gynecol Pelvic Med 2020;3:33.