Surgical technique of robot-assisted laparoscopic sacrocolpopexy
Introduction
There is no doubt, that robotic surgery rapidly spreading over many countries (1). Number of robot-assisted (RA) interventions overcame 1 million in 2018 and it’s only growing.
Robotic surgery offers many advantages compared to open surgery, including:
- 3D high definition camera with integrated lights and automated system of focus and visualization, providing a better view of the surgical site than would be available otherwise, even during open surgery.
- The robot’s “hands” have a high degree of dexterity and accuracy, allowing surgeons the ability to operate in very tight spaces in the body that would otherwise only be accessible through open (long incision) surgery (2).
- Minimization direct contacts with patients reduce the risk of bloodborne infection transmitting.
- Robotic surgery facilitates operating technique in obese patients (3-5).
- Robots cause less exhaustion for the attending surgeon during an intervention, especially ones that take multiple hours (6,7).
Apical support is paramount in the surgical treatment of pelvic organ prolapse (8). Sacrocolpopexy (SCP) remains one of the most beneficial techniques in apical prolapse treatment. It is intended to address I and II levels of support according to DeLancey, making it anatomically and pathogenetically reasonable (9).
Laparoscopic SCP despite its efficiency has some disadvantages: long learning curve, surgeon hands’ restraint, lack of tactile sensing. These factors impact on operating time, limiting wide apply of the laparoscopic approach. Robotic surgery is more relevant for SCP, because of the necessity to approach hard-to-reach pelvic spaces, a significant amount of suturing and extended operating time.
SCP indications
Apical II–IVth grade genital prolapse according to Pelvic Organ Prolapse Quantification (POP-Q) with or without concomitant rectocele. It’s more preferable in young and sexually active patients.
Surgical technique of RA-SCP
The patient is placed in a lithotomy position. Foley catheter. Left side docking.
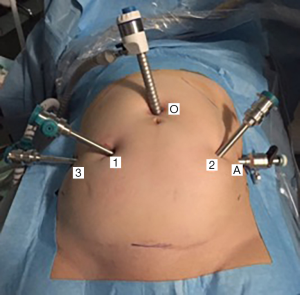
Trocar placement is aimed to reach a maximal range of robotic hands’ movements to prevent their intraoperative “conflict”. Optical trocar (12 mm) is placed at standard position—2 cm above the umbilicus. The first two robotic ports are placed laterally as possible to optical, at least 10 cm far from each other. Third robotic and laparoscopic ports are placed laterally to previous ones. Operation is carried out with three robotic ports (8 mm) and one laparoscopic (11 mm) for hand-held manipulators (suction-irrigation, grasper), mesh and suture insertion (Figure 1).
These are robotic instruments applied for SCP: bipolar forceps, monopolar scissors, grasper, long needle driver and suture cut needle driver. For vaginal cuff rectal probe is used and uterine manipulator is for the cervix.
Surgical technique of RA SCP can be divided into 4 steps.
I step
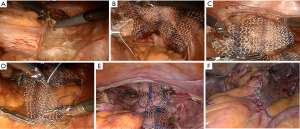
Parietal peritoneum is widely opened from cul-de-sac to the promontorium medially to sigmoid mesentery (Figure 2). Preliminary, identification of most important anatomical landmarks (right ureter, right common and internal iliac artery, median sacral vein and artery) should be provided. After that, blunt dissection of rectovaginal space to peritoneal body is made for distinguishing pubococcygeus muscles from both sides and rectovaginal septum in the middle. The anterior vaginal wall is dissected from the bladder until its middle third with preservation of pubocervical ligaments. If the patient has a uterus, then subtotal hysterectomy should be done after this step.
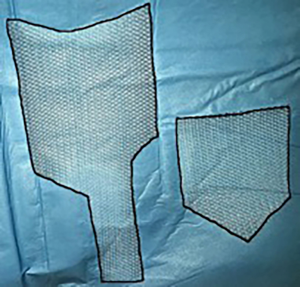
II step
Two grafts should be cut out of polypropylene material with soft index. The grafts should be made in a special shape: the posterior side size is 15 cm × 8 cm and the anterior one is 5 cm × 3 cm (Figure 3). Using braided non-absorbable suture material (Ethibond™) the edges of the 1st graft are fixed with both sides to m. pubococcygeus (part of m. levator ani) (Figure 2B). Note, that fixation is made superficially, up to 5 mm deep, because of the pudendal nerve’s risk of injury, which is located in the ischiorectal fossa’s lateral wall in the fascia of internal obturator muscle (Alcock’s canal). The edge of the 1st graft is also fixed to the uterosacral ligament as well as to the posterior surface of the cervical stump or vaginal vault. The fixation of the 2nd graft’s edge is made to the anterior vaginal wall aimed to correct cystocele, anterior surface of the cervical stump or vaginal vault (Figure 2C). The fixation is also made to the 1st graft with separate nonabsorbable sutures. The approximate distance between stitches at the anterior and posterior vaginal wall should be 2.5 and 3.5 cm respectively.
III step
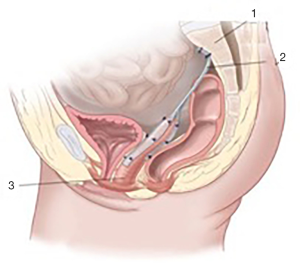
Loose end of the 1st graft being tension-free is fixed to the longitudinal presacral ligament (Figure 2D). Thus, the prosthesis becomes Y-shape finally (Figures 2E,4).
IV step
Peritoneal closure is made by the continuous suture providing resulting extraperitoneal location of mesh (Figure 2F). At the end of operation vaginal packing is obligatory.
RA SCP steps are shown in Figure 2.
Comments
From January 2013 to December 2019, 181 patients underwent RA SCP in our department. Mean operative duration was 158±37.27 min (95% CI, P<0.05). All patients have been examined at the outpatient department during 1 year after surgery. Long-term assessment (more than 1 year) was made in 74 patients. There weren’t found graft-related complications requiring additional surgery.
Subjective outcomes evaluated using the Pelvic Floor Distress Inventory (PFDI-20), Pelvic Floor Impact Questionnaire (PFIQ-7) and Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12) scores. Seventy (94.6%) of patients claimed excellent and good subjective results showing improvement in scoring.
Anatomical outcomes evaluated using POP-Q system. Sixty-one (82.4%) showed good and excellent anatomical results showing an absence of prolapse or stage I prolapse recurrence. However, 13 (17.6%) patients showed recurrent II–III stage of recurrent prolapse and all of them showed cystocele. Only 2 (2.7%) of them underwent POP surgery once more (recurrent laparoscopic SCP and vaginal mesh repair using OPUR kit).
Conclusion: RA SCP is the operation of choice in sexually active women with apical prolapse. Robotic surgery has advantages comparing laparoscopic approach in obese patients, women with dense or strong intraabdominal adhesions, recurrent pelvic organ prolapse after laparoscopic or vaginal mesh repair.
Discussion
Question 1 Dr. Liliana Mereu: do the authors find any contraindications to perform robotic SCP?
While some authors refer to adhesions and obesity as contraindications for robotic surgery, we, on the contrary, perform it mostly in obese patients, which could be a problem during a laparoscopic approach. Also, robotic surgery is very helpful for dealing with pelvic adhesions providing a better and precise view and instruments’ freedom. But, if your patient has the intraabdominal adhesive disease, we would recommend the use of Palmer’s point and open-entry Hasson technique. For absolute contraindications, we consider such as for laparoscopic approach, including acute infections, hemodynamic instability, general anaesthesia contraindications.
Question 2 Dr. Liliana Mereu: considering the various different approaches (vaginal, minimal invasive, fascial, protesic) for prolapse correction, do the authors predilige SCP in all cases of apical defect?
We agree with the widespread opinion of SCP as a “gold standard” for apical prolapse, but in case of an apical defect in reproductive age, we recommend sacrospinous fixation combined with native tissue repair techniques in cases when the cervix is remaining. We believe that in reproductive women presenting prolapse, anterior sacrospinous fixation could provide quite good anatomical results preventing mesh-associated complications and dyspareunia and only in case of recurrence SCP should be considered.
Question 3 Dr. Liliana Mereu: considering FDA alert on MESH use, did the authors change any of their surgical indications?
Nowadays many countries and gynaecologists are collecting their surgical outcomes for revision of surgical tactics for prolapse reduction, we are also collecting our outcomes too. In the Russian Federation, there is no such oblige restriction in mesh use, but we agree that every mesh-using surgery should be argued. We imply that mesh SCP should be used in recurrent patients with apical or posterior-apical prolapse with grades III–IV according to POP-Q system, also it is a method of choice in post-hysterectomy patients because of technical difficulties of grounding apex during vaginal techniques such as sacrospinous fixation or vaginal mesh surgery. Also, we insist, that anti-stress correction at the same time, especially using vaginal tapes for SUI, should be performed only in women with developed stress incontinence and not in patients with “occult” SUI. All patients that underwent mesh-using surgery should be observed more thoroughly during the first year after the operation and at least one time a year later. This recommendation helps to prevent and treat any post-op complications as early as possible and higher patient-surgeon’s communications.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Mereu Liliana) for the series “Robotic surgery for benign and malignant gynecological diseases” published in Gynecology and Pelvic Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-4/coif). The series “Robotic surgery for benign and malignant gynecological diseases” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Intuitive Surgical Inc. Annual Report 2019. Available online: http://investor.intuitivesurgical.com/static-files/ae564df0-fff1-42d9-a85f-5ef54ba22419
- Geisler JP, Orr CJ, Khurshid N, et al. Robotically assisted laparoscopic radical hysterectomy compared with open radical hysterectomy. Int J Gynecol Cancer 2010;20:438-42. [Crossref] [PubMed]
- Geppert B, Lonnerfors C, Persson J. Robot-assisted laparoscopic hysterectomy in obese and morbidly obese women: surgical technique and comparison with open surgery. Acta Obstet Gynecol Scand 2011;90:1210-7. [Crossref] [PubMed]
- George A, Eisenstein D, Wegienka G. Analysis of the Impact of Body Mass Index on the Surgical Outcomes after Robot-Assisted Laparoscopic Myomectomy J Minim Invasive Gynecol 2009;16:730-3. [Crossref] [PubMed]
- Burke WM, Gossner G, Goldman NA. Robotic surgery in the obese gynecologic patient. Clin Obstet Gynecol 2011;54:420-30. [Crossref] [PubMed]
- Boggess JF, Gehrig PA, Cantrell L, et al. A comparative study of 3 surgical methods for hysterectomy with staging for endometrial cancer: robotic assistance, laparoscopy, laparotomy. Am J Obstet Gynecol 2008;199:360.e1-9. [Crossref] [PubMed]
- Lim PC, Crane JT, English EJ, et al. Multicenter analysis comparing robotic, open, laparoscopic, and vaginal hysterectomies performed by high-volume surgeons for benign indications. Int J Gynaecol Obstet 2016;133:359-64. [Crossref] [PubMed]
- Lee RK, Mottrie A, Payne CK, et al. A review of the current status of laparoscopic and robot-assisted sacrocolpopexy for pelvic organ prolapse. Eur Urol 2014;65:1128-37. [Crossref] [PubMed]
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol 1992;166:1717-24. [Crossref] [PubMed]
Cite this article as: Popov A, Klyushnikov I, Idashkin A. Surgical technique of robot-assisted laparoscopic sacrocolpopexy. Gynecol Pelvic Med 2020;3:9.