Primary endometrioid adenocarcinoma arising on vaginal endometriosis: a case report and review of the literature
Highlight box
Key findings
• This study demonstrated the feasibility of minimally invasive surgery to treat patients with primary endometrioid adenocarcinoma arising from vaginal endometriosis.
What is known and what is new?
• Vaginal malignancies arising on endometriosis represent an extremely rare disease and it is difficult to establish a standard treatment. The results of the studies included in the review are characterized by heterogeneity, but the surgical excision represents the most common treatment.
• The laparoscopic approach may be a viable option to perform surgery in patients with primary vaginal endometrioid carcinoma arising on endometriosis.
What is the implication, and what should change now?
• Laparoscopy might represent an option when choosing the approach to perform surgery in patients with primary endometrioid adenocarcinoma arising from vaginal endometriosis.
Introduction
Endometriosis is a benign, chronic, inflammatory disease affecting approximately 10–15% of women in reproductive age, often leading to significantly impaired quality of life (1). Although it is a benign disease and it is not characterized by uncontrolled lesion growth, endometriosis, and particularly ovarian endometriosis, may increase the risk of developing malignancy (2). It is possible to identify several features involving both endometriosis and cancer, such as the development of local and distant foci, resistance to apoptosis and invasion of other tissues with subsequent damage to the target organs (3). The malignant transformation of ovarian endometriosis was initially described by Sampson in 1925 (4). In the following decades several reviews have been published, describing the risk of malignant transformation in patients with ovarian and extragonadal endometriosis.
The primary invasive vaginal carcinoma represents only 1–2% of all gynecological malignancies. More than 80% of primary vaginal malignancies are represented by squamous cell carcinoma at final histology; adenocarcinoma represents the second most common subtype, accounting for 15% of all primary vaginal cancer (5). In the last decades, several cases of endometrioid adenocarcinoma arising from vaginal endometriosis have been reported in literature.
The present study reports the case of a 59-year-old patient with a history of recurrent endometriosis and malignant transformation of a vaginal endometriotic nodule. A radical surgical treatment was performed, and adjuvant therapy was administered. A systematic review of the literature was conducted to analyze evidence about malignant transformation of vaginal endometriosis and about the related treatment options. The purpose of this review is to identify the best therapeutic strategy in patients with primary vaginal endometrioid carcinoma arising on endometriosis. We present this article in accordance with the PRISMA reporting checklist and CARE reporting checklist (available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-33/rc).
Methods
The case of a patient with endometrioid adenocarcinoma arising from endometriotic lesion of the vagina is here presented. A detailed description of the surgical procedure has been reported. Patient characteristics and surgical outcomes were registered. Oncological outcomes refer to a 36-month follow-up period. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this manuscript and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
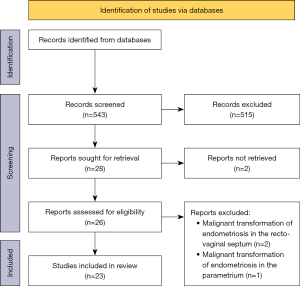
A systematic review of the literature was conducted in accordance with the methods proposed by the PRISMA guidelines (6) (Figure 1). It was performed on Ovid MEDLINE, EBM Reviews Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews from 1946 to May 2023 using the keywords “endometriosis, endometrioid” and “malignant transformation” or “carcinoma, tumor, neoplasm” and “vagina, recto-vaginal”. Search structures and keywords were tailored by a medical research librarian, specialized in systematic review and meta-analysis.
There were 543 studies meeting the search criteria. The selection of the articles was performed by two clinicians who independently screened titles and abstracts of the articles to identify relevant studies and subsequently performed a detailed review of the included articles. Only case reports and case series were included in the review. Reports and reviews of carcinoma arising in the uterus or in the ovaries were excluded, as well as those arising on bowel, ureter or bladder and surgical scars. Reports focusing only on genetic mutations or on the pathogenesis of the malignant transformation were ruled out from the review. Disagreement was resolved by consensus.
A full-text review of the 26 studies identified as eligible for the review was performed. Three articles focused on malignant transformation of endometriosis in the recto-vaginal septum or on parametria were therefore ruled out.
Eventually, 23 studies met the eligibility criteria and were included in the systematic review (7-29).
From all eligible articles, the following data were extracted: author and year of publication, patient age, menopausal status and gynecologic history, tumor histology, the therapeutic strategy adopted for each patient, either medical or surgical, and the oncological outcomes.
Extracted data were collected and analyzed; no further statistical analysis was performed.
Results
Case report
A 59-year-old patient referred to Women’s and Children “Filippo Del Ponte” Hospital, University of Insubria, Varese, Italy for abnormal genital bleeding. She had no pertinent medical history, and her family history was negative for malignancies. The diagnosis of deep infiltrating endometriosis was made in 2013, and the patient previously underwent a laparotomic oophorectomy for an endometriotic cyst. After that, she had a pregnancy and underwent a cesarean section. When she was 48 years old, she entered menopausal status and started a 10-year hormone replacement therapy.
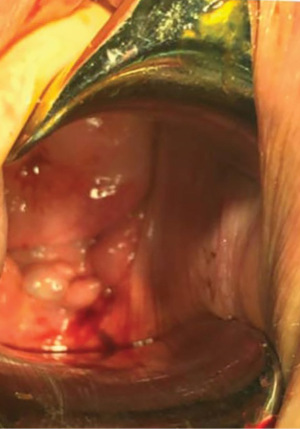
A colposcopy was performed with abnormal findings. An exophytic lesion arising on the right posterolateral vaginal fornix, suspicious for malignancy, was observed (Figure 2). A transvaginal biopsy of the lesion was performed. The nodule histologic evaluation revealed an adenocarcinoma with the following features: estrogen and progesterone receptor expression, p16+, p53+, vimentin−, WT1−, MIB1 30%, high risk human papillomaviruses (HPV) absent.
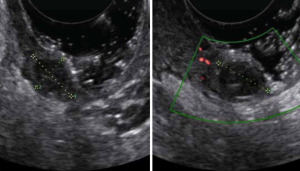
A subsequent transvaginal tenderness-guided ultrasound examination with color Doppler showed the presence of an ipoechoic mass of 30 mm × 14 mm × 28 mm (Figure 3). Magnetic resonance imaging (MRI) confirmed the ultrasound findings, showing a solid mass of 33 mm × 15 mm × 30 mm in correspondence of the right posterolateral vaginal fornix.
A nerve-sparing laparoscopic procedure was performed: total extra fascial hysterectomy with bilateral salpingo-oophorectomy and en-bloc posterior upper colpectomy was accomplished to eradicate the pathologic nodule. No other endometriotic foci were identified during the surgical procedure. Pelvic and para-aortic lymphadenectomy was not performed at the time of surgery due to the low level of evidence supporting the need for this procedure in the case of isolated vaginal adenocarcinoma on preoperative MRI assessment. The operative time was 167 min, no intraoperative complications occurred and no conversion to open surgery was required. The estimated intraoperative blood loss was 200 mL, and no blood transfusion was needed. The patient’s postoperative course was uneventful, with a hospital stay of 4 days. No postoperative complications within 30 days from surgery occurred.
The surgical specimen final pathological analysis confirmed a grade 2 endometrioid adenocarcinoma of the vagina. The vaginal wall, the posterior fibroadipose tissue and the cervical stroma were infiltrated, but the vaginal margins were free of disease. Focal images of neuroinvasion were detected within the fibroadipose tissue and the cervical stroma, while there was no evidence of lympho-vascular space invasion. Metastatic foci were observed in a small lymph node within the paracolpium, thus the tumor was graded as pTNM pT2 pN1.
Considering the histologic findings and the risk factors for recurrence, after a multidisciplinary discussion of the case, the patient was scheduled for adjuvant therapy. Chemotherapy with a platinum-taxane regimen was administered for six courses, without notable toxicity and with a clinical and radiological complete response.
Both 36-month clinical and imaging follow-up were uneventful.
Systematic review
The systematic review identified 543 studies. Reports and reviews on carcinomas arising in uterus, ovaries, bowel, ureter, and bladder or surgical scars were excluded. We also excluded reports focusing only on genetic mutations or on the pathogenesis of endometriosis malignant transformation. A total of 23 eligible studies were identified and included in the systematic review (7-29). In case of confined vaginal disease, exclusive surgical treatment was reported in 7 (30.4%) studies, while surgery followed by chemotherapy was reported as an effective treatment in 10 (43.5%) studies. Five (21.7%) studies suggested external beam radiation therapy as adjuvant option. In 3 (13%) cases, exclusive chemo-radiation treatment was proposed. The clinical findings of each study are presented in detail in Table 1.
Table 1
| Author | Year | Age | Menopause | HRT | Gynecologic history | Histology | Surgical treatment | CHT | RT | Recurrence | Follow up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ulbright T (29) | 1981 | 32 | No | No | NR | Endometrial stromal sarcoma | Vaginal nodule excision, TAH + BSO | No | No | No | 3 years |
| Kapp DS (17) | 1982 | 35 | No | No | TAH + BSO | Endometrioid adenocarcinoma G1 | NR | No | Yes | No | 7 years |
| Haskel S (7) | 1989 | 53 | Yes | N/A | – | Endometrioid adenocarcinoma G1 | TAH + BSO + pelvic and paraaortic lymph node sampling, omental biopsy, partial colpectomy | No | Yes | No | 24 months |
| McCluggage WG (20) | 1996 | 39 | Yes | Yes | TAH + right ovarian cystectomy | Endometrial stromal sarcoma G1 | Partial vaginectomy, bilateral oophorectomy and partial omentectomy | No | No | No | 3 months |
| Judson PL (16) | 2000 | 42 | No | No | TAH, appendectomy + BSO + sigmoid resection, colostomy and vaginal resection | Adenosarcoma | Exploratory LPT: colostomy, vaginal resection | Yes | No | Yes | 3 years |
| Eckert R (13) | 2000 | 68 | Yes | Yes | TAH + BSO 1972 | Endometrioid adenocarcinoma G2 | Resection of nodule + pelvic lymphadenectomy | N/A | N/A | N/A | N/A |
| Lavery S (19) | 2001 | 50 | Yes | Yes | VH + BSO | Adenocarcinoma | Resection of nodule, sigmoid resection | N/A | N/A | N/A | N/A |
| 53 | Yes | Yes | TAH + BSO | Adenocarcinoma G2 | Exploratory laparotomy: biopsies | N/A | N/A | N/A | N/A | ||
| Liu L (9) | 2003 | 56 | Yes | Yes | TAH + BSO | Mullerian adenosarcoma | Exenteration, lymphadenectomy | Yes | Yes | No | 3 years |
| Soliman NF (26) | 2004 | 60 | Yes | Yes | TAH + BSO | Endometrioid adenocarcinoma G2 | Exploratory laparotomy | No | Yes | No | 4 years |
| 51 | Yes | Yes | TAH + right SO | Endometrioid adenocarcinoma | Excision of the mass, vaginal vault, right nephroureterectomy and left SO | No | No | No | 2 years | ||
| Zorzi C (30) | 2015 | 58 | Yes | N/A | TAH, BSO, right ureteral resection and ureteroneocystostomy | Adenosarcoma | Laparoscopic radical colpotomy, with left parametrectomy and hemi-cystectomy | N/A | N/A | N/A | N/A |
| Mahdavi A (10) | 2006 | 52 | Yes | No | Laparoscopic treatment of endometriosis | Clear cell adenocarcinoma | LARVH, radical upper vaginectomy, bilateral! pelvic lymphadenectomy, lysis of pelvic adhesions, and insertion of ureteral catheters | Preoperative concomitant adjuvant CHT/RT | Preoperative concomitant CHT/RT | Yes | 8 months |
| Shah C (25) | 2006 | 55 | Yes | No | NR | Clear cell adenocarcinoma | Radical hysterectomy with upper vaginectomy, BSO, pelvic lymphadenectomy | Yes | Yes | No | 7 months |
| Nomura S (22) | 2006 | 40 | Yes | Yes | Right SO, TAH + left SO | Endometrioid adenocarcinoma G2 | NR | Yes | Yes | DOD | 16 months |
| Rachaneni S (23) | 2007 | 43 | Yes | Yes | Right SO, TAH + left SO | Carcinosarcoma | Radical upper vaginectomy plus anterior resection and removal of the rectosigmoid colon and temporary ileostomy | Yes | No | No | N/A |
| Fruscio R (14) | 2008 | 40 | No | No | NR | Endometrioid adenocarcinoma | Radical hysterectomy, BSO, bilateral pelvic and lomboaortic lymph node sampling and resection of the left vaginal wall | NACT | No | No | 24 months |
| Nomoto K (31) | 2010 | 57 | Yes | No | Laparotomic lysis of adhesions, TAH + right SO | Endometrioid adenocarcinoma | Abdominal colectomy, left SO, pelvic lymphadenectomy | No | No | No | 1 year |
| Han X (15) | 2010 | 34 | No | No | TAH +ovarian cystectomy, right SO | Adenosarcoma G1 | NR | Yes | No | No | 6 years |
| Wang S (27) | 2013 | 34 | Yes | Yes | TAH + right ovarian cystectomy + resection of rectovaginal septal lesion + partial colectomy + right SO | Mullerian adenofibroma with low grade adenosarcoma | Vaginal nodule excision | Yes | No | No | 2 years |
| Sanverdi I (12) | 2016 | 46 | No | No | NR | Endometrial stromal sarcoma | TAH + BSO, partial colpectomy | N/A | N/A | N/A | N/A |
| Pontrelli G (11) | 2016 | 58 | Yes | Yes | Laparoscopic lysis of adhesions, TAH, BSO + appendectomy | Adenosarcoma with sarcomatous overgrowth | Laparoscopic total colpectomy, parametrectomy and partial cystectomy | No | No | No | 1 year |
| Kondo E (18) | 2018 | 52 | Yes | N/A | Right SO, TAH + left SO | Endometrioid adenocarcinoma | Rejected by the patient | Yes | No | Yes | 11 years |
| Sarivalasis A (32) | 2018 | 56 | Yes | No | BSO | Endometrioid adenocarcinoma G2 | Laparoscopic posterior exenteration, pelvic lymphadenectomy | Yes | Yes | No | 1 year |
| Kim JH (8) | 2021 | 40 | No | No | NR | Complex hyperplasia and endometrial cancer | Rectovaginal mass excision + radical hysterectomy + rectum anterior resection | No | No | No | 8 months |
HRT, hormone replacement therapy; CHT, chemotherapy; RT, radiotherapy; NR, not reported; TAH, total abdominal hysterectomy; BSO, bilateral salpingo-oophorectomy; N/A, not available; LPT, laparotomy (open surgery); VH, vaginal hysterectomy; SO, salpingo-oophorectomy; LARVH, laparoscopic assisted radical vaginal hysterectomy; DOD, died of disease; NACT, neo-adjuvant chemotherapy.
Discussion
In the current study, we have elucidated the clinical presentation of a patient with a primary endometrioid adenocarcinoma originating from vaginal endometriosis. She underwent a laparoscopic total hysterectomy with bilateral salpingo-oophorectomy and en-bloc posterior upper colpectomy. Subsequently, an adjuvant chemotherapy regimen was administered. The patient exhibited a smooth postoperative recovery, and a 36-month clinical surveillance period yielded unremarkable findings, thereby underscoring the efficacy and viability of the laparoscopic surgical approach in this clinical scenario. Primary vaginal adenocarcinoma represents a rare condition that typically develops on a vaginal endometriotic nodule.
Over the past few decades, substantial research efforts have been dedicated to comprehending the transition process from typical to atypical endometriosis, culminating in malignancy. This progression has been extensively documented in the context of endometriosis-linked ovarian cancer. Back in 1925, Sampson pioneered the articulation of specific criteria for characterizing the malignant metamorphosis of endometriosis. Throughout this process, the atypical endometriosis is considered an intermediate histological condition between benign and malignant conditions (33).
The frequency of endometriosis malignant transformation is still unknown, but it is estimated that up to 1% of women with endometriosis will develop endometriosis-associated malignancies (34). The development of endometriosis-associated cancer is not yet fully understood. Menopausal status, the increasing prevalence of obesity among women, hyperestrogenism, and unopposed estrogen hormone replacement therapy seem to be relevant risk factors (19). Hormonal treatment may represent a potential risk factor for the causal pathway of cancer in endometriosis. When comparing the effects of risk factors for ovarian cancer in women with and without endometriosis, long-term menopausal estrogen-only therapy use showed an increased risk of ovarian cancer. Furthermore, the risk appeared to be greater for women with endometriosis than for those without (35). Unfortunately, there is not enough data available on the variation of cancer risk when hormonal treatments are initiated during perimenopause (36). Inflammation and reactive oxygen species associated with endometriosis may be the first step in DNA damage and cancer initiation. Additionally, mutations in genes such as K-RAS, PTEN, and ARID1A may increase the risk of endometriosis-associated cancers in young women (36,37). However, the malignant transformation of extragonadal endometriosis represents an uncommon event. Due to its rarity, it is difficult to establish the best treatment options. Overall, in the management of confined vaginal adenocarcinomas arising from endometriosis, comprehensive treatment including surgery, chemotherapy, and radiation therapy must be considered.
In Table 1, data of similar cases described in the literature are reported. Sixteen patients were in menopausal status (69.6%) and in 9 (39.1%) cases they took hormone replacement therapy; the youngest patient was 32 at the moment of the diagnosis. The most frequent symptoms reported were vaginal bleeding and pelvic pain. In the review, 14 patients were affected by cancers of epithelial origin (10 endometrioid carcinoma; 2 clear cell carcinoma; and 2 adenocarcinoma not specified), while 9 by cancers of sarcomatous origin. The investigators have proposed different options for the treatment of these patients: exclusive surgical treatment was reported in 7 (30.4%) studies, while surgery followed by chemotherapy was reported as effective treatment in 10 (43.5%) studies. Five (21.7%) studies suggested external beam radiation therapy as adjuvant option. In 3 (13%) cases exclusive chemo-radiation treatment was proposed.
Given the poorly experience with endometriosis associated vaginal adenocarcinoma, the best management is still poorly unclear. Surgical excision of malignancy represents the most common option. Regarding the surgical method, conventionally, the treatment of vaginal carcinoma often involved a comprehensive approach combining laparotomy with the vaginal route.
Nevertheless, in recent decades, laparoscopy has undergone a profound transformation in the landscape of contemporary surgery, ascending to a prominent position and emerging as the preferred method for numerous gynecologic procedures. When compared with open approach, the advantages of minimally invasive surgery have been well demonstrated by several studies: lower blood loss, less postoperative pain and perioperative complications, shorter hospitalization, better cosmetic results, and improved quality of life. Furthermore, the adjuvant therapy need and composition are not standardized, depending on patients and tumour characteristics.
Conclusions
Vaginal adenocarcinoma arising on endometriosis represents an extremely uncommon disease. Due to its rarity, it is difficult to establish a standard treatment. The results of the studies included in the review are characterized by heterogeneity, but the surgical excision represents the most common treatment. When surgery was performed, patients with malignant transformation of extragonadal endometriosis had a favorable prognosis. This study showed the feasibility of the laparoscopic approach to perform surgery. Once surgery is indicated, it is very important to perform a total excision of endometriosis lesions, because the presence of residual endometriosis, although rarely, might represent a risk factor for the development of the same malignancy. Adjuvant chemotherapy or radiation therapy may represent an option in relation with the characteristics of the patients and the extension of the disease.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist and CARE reporting checklist. Available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-33/rc
Peer Review File: Available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-33/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-33/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this manuscript and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Parazzini F, Esposito G, Tozzi L, et al. Epidemiology of endometriosis and its comorbidities. Eur J Obstet Gynecol Reprod Biol 2017;209:3-7. [Crossref] [PubMed]
- Kvaskoff M, Mahamat-Saleh Y, Farland LV, et al. Endometriosis and cancer: a systematic review and meta-analysis. Hum Reprod Update 2021;27:393-420. [Crossref] [PubMed]
- Kvaskoff M, Mu F, Terry KL, et al. Endometriosis: a high-risk population for major chronic diseases? Hum Reprod Update 2015;21:500-16. [Crossref] [PubMed]
- Sampson JA. Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. Arch Surg 1925;10:1-72. [Crossref]
- Creasman WT, Phillips JL, Menck HR. The National Cancer Data Base report on cancer of the vagina. Cancer 1998;83:1033-40. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [Crossref] [PubMed]
- Haskel S, Chen SS, Spiegel G. Vaginal endometrioid adenocarcinoma arising in vaginal endometriosis: a case report and literature review. Gynecol Oncol 1989;34:232-6. [Crossref] [PubMed]
- Kim JH, Song SH, Kim G, et al. The multistep process of vaginal cancer arising from deep infiltrating endometriosis: a case report. BMC Womens Health 2021;21:271. [Crossref] [PubMed]
- Liu L, Davidson S, Singh M. Müllerian adenosarcoma of vagina arising in persistent endometriosis: report of a case and review of the literature. Gynecol Oncol 2003;90:486-90. [Crossref] [PubMed]
- Mahdavi A, Shamshirsaz AA, Peiretti M, et al. Laparoscopic management of vaginal clear cell adenocarcinoma arising in pelvic endometriosis: case report and literature review. J Minim Invasive Gynecol 2006;13:237-41. [Crossref] [PubMed]
- Pontrelli G, Cozzolino M, Stepniewska A, et al. Primary Vaginal Adenosarcoma With Sarcomatous Overgrowth Arising in Recurrent Endometriosis: Feasibility of Laparoscopic Treatment and Review of the Literature. J Minim Invasive Gynecol 2016;23:833-8. [Crossref] [PubMed]
- Sanverdi I, Temizkan O, Vural F, et al. Primary vaginal endometrial stromal sarcoma associated with endometriosis: a case report with a review of the literature. Eur J Gynaecol Oncol 2016;37:717-21. [PubMed]
- Eckert R, Eckert R. Adenocarcinoma arising in endometriosis. Am Fam Physician 2000;62:734, 736.
- Fruscio R, Padula F, Mancini E, et al. Malignant transformation of vaginal endometriosis treated with neoadjuvant chemotherapy and surgery. J Obstet Gynaecol Res 2008;34:706-8. [Crossref] [PubMed]
- Han X, Leng J, Guo L, et al. Vaginal adenosarcoma arising from refractory endometriosis: a case report. Aust N Z J Obstet Gynaecol 2010;50:574-6. [Crossref] [PubMed]
- Judson PL, Temple AM, Fowler WC Jr, et al. Vaginal adenosarcoma arising from endometriosis. Gynecol Oncol 2000;76:123-5. [Crossref] [PubMed]
- Kapp DS, Merino M. LiVolsi V. Adenocarcinoma of the vagina arising in endometriosis: long-term survival following radiation therapy. Gynecol Oncol 1982;14:271-8. [Crossref] [PubMed]
- Kondo E, Maki S, Nii M, et al. Long-term survival of a patient with malignant transformation of extragonadal endometriosis treated solely with chemotherapy: A case report. J Obstet Gynaecol Res 2018;44:2186-9. [Crossref] [PubMed]
- Lavery S, Gillmer M. Malignant transformation of residual endometriosis in women on unopposed oestrogen hormone replacement therapy. BJOG 2001;108:1106-7. [Crossref] [PubMed]
- McCluggage WG, Bailie C, Weir P, et al. Endometrial stromal sarcoma arising in pelvic endometriosis in a patient receiving unopposed oestrogen therapy. Br J Obstet Gynaecol 1996;103:1252-4. [Crossref] [PubMed]
- Nomoto K, Hori T, Kiya C, et al. Endometrioid adenocarcinoma of the vagina with a microglandular pattern arising from endometriosis after hysterectomy. Pathol Int 2010;60:636-41. [Crossref] [PubMed]
- Nomura S, Suganuma T, Suzuki T, et al. Endometrioid adenocarcinoma arising from endometriosis during 2 years of estrogen replacement therapy after total hysterectomy and bilateral salpingo-oophorectomy. Acta Obstet Gynecol Scand 2006;85:1019-21. [Crossref] [PubMed]
- Rachaneni S, Spencer C, Lloyd J, et al. Malignant transformation of post-hysterectomy vault endometriotic nodule. J Obstet Gynaecol 2007;27:434-7. [Crossref] [PubMed]
- Costanza M, Herrera F, Hastir D, et al. A Locally Advanced Endometrioid Adenocarcinoma Arising from Vaginal Endometriosis: Management and Review of the Literature. Reports 2021;4:29. [Crossref]
- Shah C, Pizer E, Veljovich DS, et al. Clear cell adenocarcinoma of the vagina in a patient with vaginal endometriosis. Gynecol Oncol 2006;103:1130-2. [Crossref] [PubMed]
- Soliman NF, Evans AJ. Malignancy arising in residual endometriosis following hysterectomy and hormone replacement therapy. J Br Menopause Soc 2004;10:123-4. [Crossref] [PubMed]
- Wang S, Lang JH, Leng JH, et al. Serum CA125 changes in one case with malignant transformation from benign endometriosis to Müllerian adenosarcoma. Chin Med J (Engl) 2013;126:3397-8. [Crossref] [PubMed]
- Chapron C. Guest Speakers Abstracts. 1st Congress of the Society of Endometriosis and Uterine Disorders. J Endometr Pelvic Pain Disord 2015;7:1-82.
- Ulbright TM, Kraus FT. Endometrial stomal tumors of extra-uterine tissue. Am J Clin Pathol 1981;76:371-7. [Crossref] [PubMed]
- Zorzi C, Mabrouk M, et al. Laparoscopic treatment of primary vaginal adenosarcoma arising in recurrent endometriosis: A case report. Journal of Endometriosis and Pelvic Pain Disorders 2015;7:S63.
- Nomoto K, Hori T, Kiya C, et al. Endometrioid adenocarcinoma of the vagina with a microglandular pattern arising from endometriosis after hysterectomy. Pathol Int 2010;60:636-41. [Crossref] [PubMed]
- Sarivalasis A, Wolfer A, et al. The case of a locally advanced endometrioid adenocarcinoma arising from vaginal endometriosis: Diagnosis and multidisciplinary treatment. International Journal of Gynecological Cancer 2018;28:25-6.
- Bartiromo L, Schimberni M, Villanacci R, et al. A Systematic Review of Atypical Endometriosis-Associated Biomarkers. Int J Mol Sci 2022;23:4425. [Crossref] [PubMed]
- Stern RC, Dash R, Bentley RC, et al. Malignancy in endometriosis: frequency and comparison of ovarian and extraovarian types. Int J Gynecol Pathol 2001;20:133-9. [Crossref] [PubMed]
- Phung MT, Muthukumar A, Trabert B, et al. Effects of risk factors for ovarian cancer in women with and without endometriosis. Fertil Steril 2022;118:960-9. [Crossref] [PubMed]
- Vercellini P, Viganò P, Buggio L, et al. Perimenopausal management of ovarian endometriosis and associated cancer risk: When is medical or surgical treatment indicated? Best Pract Res Clin Obstet Gynaecol 2018;51:151-68. [Crossref] [PubMed]
- Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med 2010;363:1532-43. [Crossref] [PubMed]
Cite this article as: Casarin J, Lembo A, Bordi G, Gisone BE, Cattelan C, Artuso V, Ghezzi F, Cromi A. Primary endometrioid adenocarcinoma arising on vaginal endometriosis: a case report and review of the literature. Gynecol Pelvic Med 2024;7:5.