Parasitic leiomyoma in the anterior abdominal wall: a case report
Highlight box
Key findings
• There were a woman with parasitic leiomyoma in the anterior abdominal wall reported after laparoscopic myomectomy.
What is known and what is new?
• In the female reproductive system, leiomyoma is the most common benign tumor, usually occurring in the uterus.
• Leiomyoma may be found in the abdominal wall with parasitic characteristic.
What is the implication, and what should change now?
• It is particularly important to completely remove the leiomyoma in surgery and carefully examine the abdomen. The appropriate extraction of the specimens should be chosen in the surgery.
Introduction
In the female reproductive system, leiomyoma is the most common benign tumor, with the incidence of 20–30% in women in the reproductive age (1). The most common site for this tumor is uterus, but it may also be found in other places, such as the ovary, the broad ligament, and in rare cases in the abdominal wall (2). To completely remove the mass is the best treatment of abdominal wall myoma in order to reduce the recurrence (3). It has been believed that implantation of myomatous tissues in the previous gynaecological surgery lead to uterine mass to adhere to adjacent organs, which then develops its own blood supply and gradually loses connection with the original organ, thus becoming a ‘parasite’ in the new location (4). Herein, a woman with parasitic leiomyoma in the anterior abdominal wall was reported in our study after laparoscopic myomectomy. We present this case in accordance with the CARE reporting checklist (available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-28/rc).
Case presentation
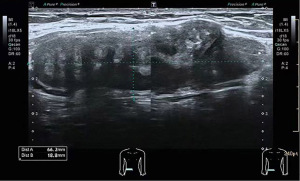
A 33-year-old Chinese woman with a laparoscopic myomectomy 8 years ago and she touched the subcutaneous mass with a diameter of 1cm at the scar of the right lower abdominal laparoscopic puncture hole after that. Then, the mass gradually increases, accompanied by menstrual pain, without skin redness, swelling, exudation, tenderness, and no changes in bowel movements. The superficial organ ultrasound examination showed that the heterogeneous weak echo mass was found in the subcutaneous layer of incision on the abdominal wall, with a size of 6.6 cm × 1.9 cm × 4.1 cm and clear boundaries, regular morphology, a fused shape, with peripheral blood flow signals detected, as shown in Figure 1. Physical examination and palpation of right Mai’s point touched a 1 cm × 2 cm spindle shaped nodule, without tenderness.
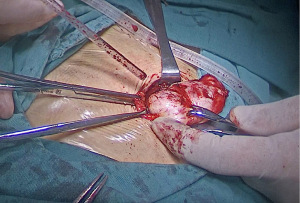
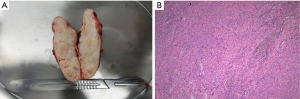
We operated the excision of the mass through abdominal wall incision. In the operation, a hard nodule was located in the anterior to posterior sheath of the muscle in the subcutaneous scar of the original laparoscopic puncture hole in the lower right abdomen, with a size of approximately 6 cm × 3 cm × 2 cm and clear boundaries with the surrounding area, as shown in Figure 2. After complete resection, it was sent for pathological examination. Upon completion of the surgery, the nodule was dissected and showed a gray white spiral shape on the section, as shown in Figure 3A. The pathologic diagnosis of the abdominal mass was leiomyoma, as shown in Figure 3B. One month later, the patient did not have any symptoms in the outpatient follow-up.
The study was approved by the institutional review board of West China Second Hospital of Sichuan University. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Leiomyomas is not limited to the uterus, but may show unusual growth patterns or in unusual position, leading their further clinical and radiological identification more difficult. The incidence of the abdominal wall leiomyoma is rare, and can be caused by the surgical removal of the uterine fibroids followed by the implantation of myometrium tissue, especially after laparoscopic myomectomy (5). Paul E. Steiner from the University of Chicago firstly reported these leiomyomas in 1939 and there are no more than 200 cases have been recorded with a median diagnostic interval of 48 months (6,7). Moon et al. reported there were three years in the formation of abdominal wall myoma after laparoscopic myomectomy (2). In our case, the woman performed a laparoscopic myomectomy 8 years ago and then found a myoma in the abdominal wall. Moreover, this myoma also occurs after transabdominal hysterectomy or without history of myomectomy (8-12). These cases indicates that this type of myoma should be considered in the differential diagnosis of patients with abdominal or pelvic solid tumors even if there is no history of laparoscopic surgery using a power morcellator.
In our previous surgery, a myoma with a diameter of approximately 1cm between the uterine anterior wall muscles and protruding beneath the serosa, was completely removed and directly taken out from the abdominal laparoscopic puncture hole without morcellation. We carefully examine the abdomen without residue after surgery. With the development of laparoscopy technology, the extraction of the specimens is more and more important. It is popular to previously use electric morcellators for extraction, however, uterine sarcoma might develop as a cause of the spread of tumor cells during morcellation when removal of the fibroids. Lieng et al. reported the incidence of unintended morcellation of a uterine sarcoma was very low (0.02%) (13). However, Perkins et al. thought that this may be more common in 1/350, although published rates range widely from 1 in 352 to 1 in 7,400 (14). Therefore, in 2014, the U.S. Food and Drug Administration (FDA) issued an advisory recommending that all information should be shared with patients when use of electric morcellators in the surgery and encouraged the gynecologists to develop new techniques (15). As a consequence, transvaginal retrieval after laparoscopic myomectomy was developed and was thought to be a valuable alternative to intracorporeal morcellation (16,17). What’s more, in-bag abdominal manual morcellation have been described for laparoscopic surgery, during which each excised myoma was placed into a sample retrieval bag, and manually morcellated with a knife or scissors via small abdominal wall incisions (17). Several studies considered that in-bag (contained) morcellation was a safe and unexpensive way to decrease the risk of dissemination, even with large specimens (18,19). It is thought that increased incisional length shortens surgical times dramatically (20,21). As the incision at the belly button can reach 2–3 cm after being opened in single-port laparoscope, the specimen is easier to take out which makes it increasingly popular in myomectomy. Moreover, adhesion and their adverse effects in later surgery can be reduced by applying antiadhesive agents during previous gynecologic surgery (22). It should be emphasized that there is one type of myomas close to the uterine cavity, the treatment is hysteroscopic myomectomy rather than laparoscopy (23). Nevertheless, more evidence is needed to assess the safety and effectiveness of hysteroscopic myomectomy and gynecologists should be trained before performing hysteroscopy in order to improve the safety (24). However, the leiomyoma was small and was not morcellated in our study. The reason for leiomyoma in the anterior abdominal wall is unexplained and it might be the parasitic.
Several theories have been proposed to explain the formation of myoma in the abdominal wall with classifying as primary and parasitic. Parasitic leiomyomas may be detected asymptomatically or unexpectedly during clinical or USG examination. The symptoms depend on its location. Abdominal pain and pelvic pressure are the most common symptoms. Other symptoms include an increase in urinary frequency, which can lead to different degrees of urinary outflow obstruction or secondary hydroureteronephrosis as a result of compression of the urethra, bladder neck or ureter (25). In our case, the patient’s abdominal wall mass was accompanied by menstrual pain, therefore, we misdiagnosed endometriosis in abdominal wall. Somatic soft tissue myoma are usually accompanied by large local masses. Macroscopically, these myomas are described as the shape of masses with clear borders that are surrounded by a fiber false capsule (26). The abdominal wall lesions and desmoid tumors are more hyperdense than leiomyoma. On the other hand, abdominal wall myoma is lentiform, while endometriosis lesions and desmoid tumors are round or oval masses (3). Before operation, we cannot distinguish the myoma with endometriosis according to the ultrasound and physical examination. The gross specimen and pathology help the diagnosis.
There are several limitations in our study. First, due to rarity of similar cases, only one case was included instead of case series. Secondly, the reason for leiomyoma in the anterior abdominal wall is unexplained because of the little samples and more data would be needed for further study. Despite these limitations, our study presents important strengths. To our best knowledge, our study provides further evidences of a rare complication after laparoscopic myomectomy. Every effort should be made to completely remove the leiomyoma and avoid morcellation to prevent implantation and recurrence.
Conclusions
The formation of myoma in the abdominal wall is rare, but considering that leiomyoma can form anywhere in the body with smooth muscles, such as the anterior abdominal wall, which should be used as a differential diagnosis of abdominal masses. To completely remove the mass is the best treatment of abdominal wall myoma in order to reduce the recurrence
Acknowledgments
Funding: This study was supported by the Science and Technology Department of Sichuan Province (grant No. 2022NSFSC1356).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-28/rc
Peer Review File: Available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-28/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-28/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional review board of West China Second Hospital of Sichuan University. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fasih N, Prasad Shanbhogue AK, Macdonald DB, et al. Leiomyomas beyond the uterus: unusual locations, rare manifestations. Radiographics 2008;28:1931-48. [Crossref] [PubMed]
- Moon HS, Koo JS, Park SH, et al. Parasitic leiomyoma in the abdominal wall after laparoscopic myomectomy. Fertil Steril 2008;90:1201.e1-2. [Crossref] [PubMed]
- Patterson GK, Winburn GB. Abdominal wall endometriomas: report of eight cases. Am Surg 1999;65:36-9. [Crossref] [PubMed]
- Al-Wadaani HA. Anterior abdominal wall leiomyoma arising de novo in a perimenopausal woman. Oman Med J 2012;27:323-5. [Crossref] [PubMed]
- Lalor PF, Uribe A, Daum GS. De novo growth of a large preperitoneal lipoleiomyoma of the abdominal wall. Gynecol Oncol 2005;97:719-21. [Crossref] [PubMed]
- Oindi FM, Mutiso SK, Obura T. Port site parasitic leiomyoma after laparoscopic myomectomy: a case report and review of the literature. J Med Case Rep 2018;12:339. [Crossref] [PubMed]
- Chan ACK, Chiu JHF, Chan YHY. Parasitic leiomyoma in the anterior abdominal wall. ANZ J Surg 2020;90:E52-3. [Crossref] [PubMed]
- Wang J, Liu G, Yang Q. Parasitic myoma after transabdominal hysterectomy for fibroids: a case report. BMC Womens Health 2023;23:310. [Crossref] [PubMed]
- Hafizi L, Pourhoseini SA. Abdominal Wall Leiomyoma: A Case Report. J Reprod Infertil 2020;21:151-4. [PubMed]
- Mousa HM, Al-Salam SA, Abdullah A, et al. Primary anterior abdominal wall leiomyoma in a pregnant woman. ANZ J Surg 2019;89:E527-8. [Crossref] [PubMed]
- Garrido Oyarzún MF, Saco A, Castelo-Branco C. Anterior abdominal wall parasitic leiomyoma: case report. Gynecol Endocrinol 2018;34:103-6. [Crossref] [PubMed]
- Ernest Ong CW, Siow SL. Case report: Leiomyoma of the anterior abdominal wall. Med J Malaysia 2016;71:81-2. [PubMed]
- Lieng M, Berner E, Busund B. Risk of morcellation of uterine leiomyosarcomas in laparoscopic supracervical hysterectomy and laparoscopic myomectomy, a retrospective trial including 4791 women. J Minim Invasive Gynecol. 2015;22:410-14. [Crossref] [PubMed]
- Perkins RB, Handal-Orefice R, Hanchate AD, et al. Risk of Undetected Cancer at the Time of Laparoscopic Supracervical Hysterectomy and Laparoscopic Myomectomy: Implications for the Use of Power Morcellation. Womens Health Issues 2016;26:21-6. [Crossref] [PubMed]
- Tinelli A, Farghaly SA. Morcellation of occulted sarcomas during laparoscopic myomectomy and hysterectomy for patients with large fibroid uterus. Minerva Ginecol 2018;70:84-8. [PubMed]
- Ghezzi F, Casarin J, De Francesco G, et al. Transvaginal contained tissue extraction after laparoscopic myomectomy: a cohort study. BJOG 2018;125:367-73. [Crossref] [PubMed]
- Glaser LM, Friedman J, Tsai S, et al. Laparoscopic myomectomy and morcellation: A review of techniques, outcomes, and practice guidelines. Best Pract Res Clin Obstet Gynaecol 2018;46:99-112. [Crossref] [PubMed]
- Devassy R, Cezar C, Krentel H, et al. Feasibility of myomatous tissue extraction in laparoscopic surgery by contained in - bag morcellation: A retrospective single arm study. Int J Surg 2019;62:22-7. [Crossref] [PubMed]
- Feghali EJ, Laganà AS, Daccache A, et al. Endobag use in laparoscopic gynecological surgeries: a systematic review. Minim Invasive Ther Allied Technol 2022;31:698-703. [Crossref] [PubMed]
- Sanderson DJ, Sanderson R, Cleason D, et al. Manual morcellation compared to power morcellation during robotic myomectomy. J Robot Surg 2019;13:209-14. [Crossref] [PubMed]
- Güven CM, Uysal D. In-bag abdominal manual morcellation versus contained power morcellation in laparoscopic myomectomy: a comparison of surgical outcomes and costs. BMC Surg 2023;23:106. [Crossref] [PubMed]
- Esber S, Etrusco A, Laganà AS, et al. Clinical outcomes after the use of anti-adhesive agents in laparoscopic reproductive surgery. Gynecol Obstet Invest 2023; Epub ahead of print. [Crossref] [PubMed]
- Etrusco A, Laganà AS, Chiantera V, et al. Feasibility and Surgical Outcomes of Hysteroscopic Myomectomy of FIGO Type 3 Myoma: A Systematic Review. J Clin Med 2023;12:4953. [Crossref] [PubMed]
- Mazzon I, Etrusco A, Laganà AS, et al. Training in Diagnostic Hysteroscopy: The "Arbor Vitae" Method. Medicina (Kaunas) 2023;59:1019. [Crossref] [PubMed]
- Lete I, González J, Ugarte L, et al. Parasitic leiomyomas: a systematic review. Eur J Obstet Gynecol Reprod Biol 2016;203:250-9. [Crossref] [PubMed]
- Goyal N, Khurana N. Leiomyoma of rectus sheath: an uncommon entity: report of two cases. Indian J Pathol Microbiol 2010;53:591-2. [Crossref] [PubMed]
Cite this article as: Yang T, Tan S, Luo L, Luo B. Parasitic leiomyoma in the anterior abdominal wall: a case report. Gynecol Pelvic Med 2023;6:29.