A step-by-step decalogue for performing a simplified type A total laparoscopic hysterectomy using fewer accesses and tools
Highlight box
Surgical highlights
• Laparoscopic hysterectomy (LH), using three laparoscopic accesses without the aid of uterine manipulators is safe and reduces the invasiveness, the costs and the number of physicians involved.
What is conventional and what is novel/modified?
• Conventionally, type A LH is performed with four laparoscopic access with a uterine manipulator.
• We use three access without uterine manipulator. To bypass these obstacles a small swab packed on a Schroeder forceps and covered by a lubricated glover is deeply inserted in the vagina to facilitate the colpotomy.
What is the implication, and what should change now?
• Performing type A hysterectomy as suggested, a decrease in the overall hospitalization, without affecting the patient’s satisfaction and safety, cost and the number of personnel used can be reached.
Introduction
Background
Hysterectomy for benign diseases embodies the most major surgical procedure performed by Gynaecologists worldwide after caesarean section (1). In 2008, Querleu and Morrow categorized extrafascial hysterectomy into four categories (2). Type A extrafascial hysterectomy is the most used for benign pathologies, ovarian and endometrial cancers and microinvasive cervical cancers (3). Different official guidelines have been published over decades to tailor the indications on the best minimally invasive, safest, and proper surgical approach (4). Hysterectomy can be performed via laparoscopic hysterectomy (LH), laparoscopic-assisted vaginal hysterectomy (LAVH), robotic hysterectomy (RH) or abdominal hysterectomy (AH). There’s a common agreement to choose the most minimally invasive approach to reach the best surgical outcomes, the less peri-operative complications, the shortest recovery, the cheapest hospital charge and not the least, the best patient fulfilment (5). Standard practice guidelines based on the Cochrane reviews define the demanding process for choosing the best surgical approach (2). In this perspective, despite LH and VH having similar outcomes, meta-analyses have shown that when both procedures are feasible, VH shows shorter operative time, lower vaginal dehiscence and conversion to laparotomy rates and lower costs (6).
Rationale
Nevertheless, LH has gained rising consent among surgeons over the last decades and, surprisingly, in 2012 the rate of performed LH overcame for the first time the rate of performed VH (7). If on one hand, vaginal dehiscence and conversion to laparotomy rates are strictly connected to the surgeon’s skill, the actual differences in costs are more challenging to assess, due to the difference between countries and, of note, due to the differences in used tools between centres. Considering this aspect, which is particularly relevant in the decision-making chart, setting an LH using the minimal numbers of ancillary trocars and choosing the right tools to perform the safely and effectively the procedure might decrease the overall hospitalization, without affecting the patient’s satisfaction and safety.
Objective
The purpose of this Decalogue is to report, step by step a type A LH. We present this article in accordance with the SUPER reporting checklist (available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-26/rc).
Preoperative preparations and requirements
Preliminary counselling process
Procedures were performed by the same surgeons (F.C. and B.C.) at two tertiary Hospitals: IRCCS “Regina Elena” National Cancer Institute, Rome, Italy and Humanitas, Istituto Clinico Catanese, Catania, Italy.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this manuscript and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
After the eligibility assessment, due to the broad range of approaches, counselling must have a prominent role in the preliminary assessment, advocating a shared and tailored decision on the patient’s characteristics and preferences. Selection of the proper procedure according to the physical patient’s characteristics is crucial to goal safe and satisfaction results. In this regard, we suggest using this modified approach in patients with good performance status without any comorbidity that could justify an inadequate Trendelenburg tilting. Each patient should be adequately informed and prepared about the offered procedure, spending adequate time in the preliminary interview to explain the procedure exhaustively. Furthermore, every alternative procedure should be exposed to encompassing all advantages and disadvantages. To enshrine the counselling process, the patient must sign an informed consent.
Patient preparation and positioning
Pre-operative bowel preparation in minimally invasive surgery has been recently arose controversial debate on its utility and outcomes despite it having been a common practice for many decades. Recent evidences suggest that the procedure do not reduce the operative time, do not improve the surgical field of view and do not decrease rates of surgical site infections, anastomotic leaks, or major morbidity (8). Patients are placed in a dorsal lithotomy position with the arms lying alongside and in gynaecological position to allow vaginal access. Trendelenburg position should only be used after the insertion of the first trocar. This position in fact raises the sacral promontory and therefore places the large vessels (aortic bifurcation and left iliac vein) on the same axis of insertion increasing the risk of vascular injury. Anti-thrombotic prophylaxis is desirable positioning pneumoboots. A foam pad is situated under the patient preventing dangerous sliding during Trendelenburg tilting. Shoulder holders can also be used to prevent patient drifting. To enhance the ergonomic working environment, the lower position of the operating table with a frontal monitor facing each surgeon is advisable. The vesical catheter should be placed before starting the procedure and will be removed at <6 hours postoperatively. The surgeon should be familiar with the equipment and should routinely inspect the equipment for any malfunction before starting the procedure. In general, it is suggested to simplify the equipment lists as much as possible preventing crowding, facilitating room turnover and ensuring the right theatre financial outlay. Since there are no trials assessing the use of prophylactic antibiotics for any type of LH it would seem reasonable to treat these patients in a similar fashion to VH. Consequentially, a single dose of first- or second-generation cephalosporin (mainly cefazoline 2 g), intravenously, 30–60 minutes before the incision is indicated. In patients with morbid obesity (body mass index >35 kg/m2), doubling the antibiotic dose may be considered (9).
Uterine manipulator positioning, our experience and suggestion
Despite several manipulators described in the literature reaching an adequate level of safety and manageability, they represent the first stumbling block in cost review. This is not only directly related to the cost of this tool but to the necessity of a second assistant that might stay sitting between the patient’s legs for the procedure. Furthermore, the uterine manipulator is not always usable. Not too far from occasionally, the vagina becomes a narrow cavity due to obesity, pelvic conformation, no sexual intercourse, and genital atrophy making impossible a safe uterine manipulator positioning. The positioning of the uterine manipulator might be contraindicated in some cases of oncological pathology. In these cases, the packaging of a vaginal manchette may be indicated to prevent tumour dissemination. In our experience, when endometrial malignancies are ruled out and when a manipulation may help the surgeons in particular steps, we bypassed these obstacles using a small swab packed on a Schroeder forceps and covered by a lubricated glover pushed deeply in the vagina. Therefore, contrary to the uterine manipulators that require the presence of a second assistant trained in the assembling and positioning process, the unique assistant can easily use their free hand to push up and lateralize the fornixs during the section of the parametrial, uterine vessels and paracervix tissues. The vaginal swab also can help during colpotomy. As further discussed below, using fewer ancillary trocars, the assistant can handle the hysterometer (when used) just pushing the uterus due to the possibility of using the right freehand. However, in our experience, we don’t use routinely this approach.
For successful and safe laparoscopic procedures an appropriate laparoscopic column is mandatory. The laparoscopic column consists of a CO2 insufflator, a monitor, a video recording system with a camera and a light generator.
Monitor, video recording system and camera
Nowadays, a broad variety of video-monitoring systems are made up of integrated and cooperating components, including a full ultra-high definition (UHD) 4K monitor, camera control unit and UHD camera. The latest cameras allow magnifying the anatomical structures and their details much better than with open surgery, letting the surgery more precise and safer. These cameras can also allow to set the color spectrum, extending beyond the human eye’s capabilities. In fact, by choosing a wide range of contrast and outstanding color reproduction, the view can be adapted for each clinical necessity such as the visualization of blood vessels and lesions. Thus, for a good resolution, a Full 4K (at least 3,840×2,160 pixel) resolution monitor is advisable. A good camera should be highly sensitive, should not have delay in transmitting images and should have good ergometry with easy button access to the multifunctional setting and the electronic zoom (10).
Laparoscopic optic
The optic is connected to the camera and to the light generator through the fibre-optic cable. There mostly are two types of laparoscopic optic, 0° and 30° optic. Generally, the most commonly used optic is 0° which directly reflects the images. In case of anatomical disruptions such as the presence of large isthmic and anterior myomas, the 30° optic could be a valuable choice because helps to catch the images bypassing the obstacle. However, specific training must be performed for mastering the correct usage. 3D optics are also available that offer a greater definition of spaces and anatomical details. Finally, infra-red filters have been developed for the identification of tracers, used mainly in oncology and recently also in the case of benign pathology.
CO2 insufflator and smoke aspirator system
CO2 is used to create pneumoperitoneum. The ideal pressure to prevent hypercarbia, acidosis, sympathetic stimulation from decreased venous return and vagal stimulation by stretching of the peritoneum shall not be above 12 mmHg (11). Ideally, the less pressure is used the fewer complications onset. Obesity or cardiovascular comorbidity claims intra-abdominal lowest pressure. However, data suggest low-pressure laparoscopy is a difficult technique to perform due to the limitations inherent in conventional insufflation procedures. For these purposes, dedicated all-in-one devices composed of insufflation, trocar access and air filtration aspirating system have been created for optimizing pneumoperitoneum. These devices electronically analyse and supply the gas flow ensuring a high insufflation flow. They can maintain a low intra-abdominal pressure without compromising exposure despite constantly removing fumes.
Trocars
We just need one 10 mm trocars and two 5 mm trocars.
The surgical instruments for this intervention are very basic:
- Atraumatic laparoscopic grasper;
- Laparoscopic myoma grasping forceps;
- Irrigation/aspiration laparoscopic system;
- Bipolar;
- Advanced electrosurgical devices (radiofrequency or ultrasound energy) for coagulation and cut;
- Laparoscopic needle driver;
- Laparoscopic knot pusher.
Studies in the literature didn’t show differences between the methods of laparoscopic access in major vascular or visceral complications (11,12). For the introduction of the Verres needle the umbilical region is preferred because the subcutaneous and preperitoneal fat is less represented, therefore the distance between skin and peritoneum is shorter. To remove the great vessels, it is recommended to pull up the abdominal wall during the penetration of the needle with the hands or with forceps for counter traction. In addition, it is advisable to empty the stomach before the insertion. Veress needle is inserted in the midline in sagittal plane at a 45-degree angle to the spine through a 3mm skin incision. To verify the correct positioning of the Verres needle in the peritoneal cavity, the most used test consists of three stages. The first step consists of aspiration with a syringe that must not produce air or liquid material suspected of vascular, intestinal, or urinary injuries. Subsequently, 20 cc of physiological solution or air is injected to verify the absence of obstacles. Finally, the lack of aspiration of the injected material allows us to confirm the correct positioning of the Verres needle. After the pneumoperitoneum is created a skin incision is performed, to introduce the 10 mm trocar. The open access consists of the incision of the skin and of the deeper tissue until you reach the peritoneal cavity. Consequently, the trocar can be safely inserted. Disposable optic trocars are available for direct insertion. They allow a continuous visualization of all the abdominal layers during trocar introduction. Once the first trocar is placed in the umbilicus for the camera, one 5 mm trocar is placed in the left iliac fossa 2–3 cm (approximately two thumbs) medial and above the left iliac spine to insert the trocar in the plica umbilicalis lateralis and laterally the inferior epigastric vessels. The other 5 mm trocar is placed in the hypogastric region, 2–3 cm above the pubic symphysis away from the bladder. For an enlarged uterus, it is useful to place the optical trocar 3–4 cm cranially. An alternative approach to the peritoneal cavity is the left subcostal place at Palmer’s point (2 cm below the costal margin along the mid-clavicular line).
Step-by-step description
Operative instruments like the aspirator, bipolar, needle driver, and electrosurgical advanced instruments should be introduced through the suprapubic trocar, however, electrosurgical might be used by lateral trocar according to the ability of the surgeon. Grasper should preferably be introduced through the lateral trocar.
Before proceeding with the description of the operative times, a clarification is mandatory.
If we want to preserve ovaries, we must start the operation from the coagulation and cut of the tubo-ovarian ligament, keeping the salpinx stretched from the fimbriae, then we proceed with the coagulation and cut of the meso-salpinx up to the utero-ovarian ligament that can be cut together with the round ligament. Following this way, ovarian suspension is not required, because ovaries maintain their physiologic position and vascularization. When both ligaments are completely sectioned, the two pages of the peritoneum can be opened next to the uterus.
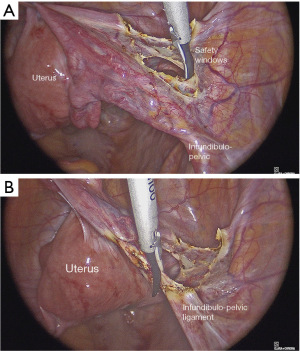
Contrary, if we decide to don’t preserve ovaries, the first surgical step is the section of the round ligament, which must be sectioned next to the umbilical artery (Figure 1A). Then we proceed with the opening of the superior page of the broad ligament, till it gets over the ovary, identifying the infundibulo-pelvic ligament. The creation of the “safety window” at the level of the posterior page of the broad ligament allows the removal of the ureter and the infundibulum-pelvic ligament coagulation in total safety (Figure 1B). Keeping stretched the adnex, we advise to uncover the infundibulo-pelvic ligament just gently cutting the peritoneum that surrounds it, to allow an efficient coagulation of ovarian vessels. Once coagulated and completely sectioned the ovarian vessels, just pulling gently the adnexes, the posterior page of the peritoneum is opened without bleeding.
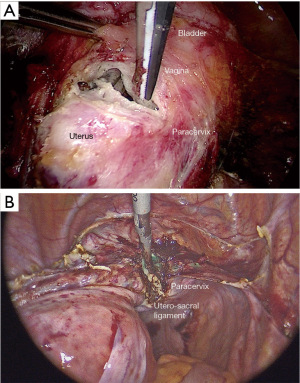
Section of the utero-sacral ligament
Pushing the tenaculum, and moving the uterus to the opposite side, we can section the utero-sacral ligament (Figure 2A) very close to the uterus and we can open the half of the peritoneum that covers the posterior part of the cervix. The same operative times must be done on the opposite side.
Vescico-uterine pouch
Moving the uterus to the opposite side, grabbing it from the round ligament, we can open the peritoneum covering the vescico-uterine pouch (Figure 2B).
Dissection of the vescico-uterine space
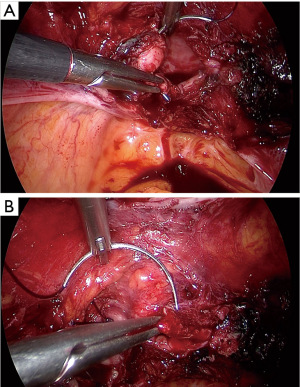
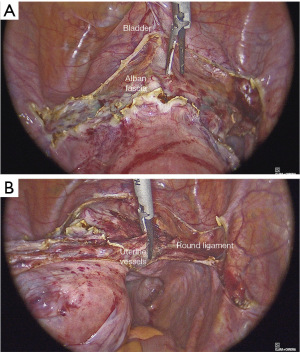
Pulling the bladder up, longitudinal connective fiberes become evident, therefore they can be sectioned with the advanced electrosurgical device (Figure 3A), up to the visualization of the cervico-vaginal fascia (Alban fascia), well evident because of its white color. At this point, maintaining pushed cranially the uterus, pushing down with the tip of the instrument, the bladder is moved and the anterior fornix is exposed. The development of the vescico-vaginal septum must always be performed before the coagulation of the uterine vessels to prevent the retraction of the tissues that could hinder these manoeuvres. During a difficult cervical-vaginal dissection, filling the bladder passively with 60 cc of diluted methylene blue staining may be helpful. This can prevent any unnoticed bladder lesions from being not recognised.
Uterine vessels
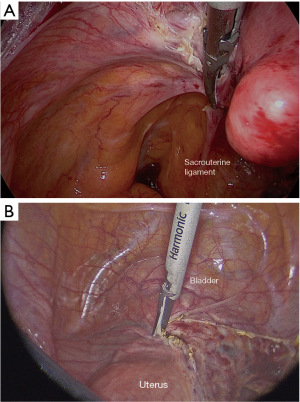
Continuing to push the uterus through the tenaculum, pulling to the opposite side, we can clamp the uterine vessels 1 cm above the limit with the bladder, thus avoiding ureter injuries (Figure 3B). We continue sectioning the cardinal ligament up to the fascia surrounding vaginal fornices is completely exposed (Figure 4A). The development of the iuxta-uterine part of medial para-rectal space (Okabayashi space) reduces the risk of ureteral injury.
Colpotomy
We advise starting colpotomy from the anterior fornix because it is easier to understand the limit between cervix and vagina, due to the presence of the gauze. Colpotomy can be done with the active branch of an advanced ultrasonic device or with a monopolar hook (Figure 4B).
Suture of the vagina
The suture can be done vaginally or laparoscopically, with a continued suture or with disconnected stitches it depends on the surgeon’s skills and preferences. We prefer to perform it laparoscopically to reduce complications such as vaginal dehiscence and vaginal infection. We advise to include in the suture, the uterosacral ligaments, to a greater suspension to the vaginal cuff (Figure 5A,5B).
Postoperative considerations and tasks
At our Institutions, we routinely use the enhanced recovery after surgery (ERAS) protocol to promote a safe and fast post-operative recovery (13). A randomized control trial, evaluating some post-operative outcomes (pain, vomiting and nausea, anesthesiologic and surgical complications up to 30 days after surgery, rate of readmissions, the time to event in hours for bowel movements, flatus, drinking, hunger, eating, and walking and the quality of recovery) had shown how the ERAS protocol enhanced recovery after surgery in gynaecologic surgery regardless of surgical access and type of gynecologic disease reducing also hospital stay and costs. Our average discharge amount in two days after the procedure (14).
Tips and pearls
Type A LH is the most used surgical approach for both benign and malignant gynaecological diseases.
- A proper pre-operative clinical evaluation, patient preparation and positioning are crucial for performing safe and effective procedures.
- Using the minimal numbers of ancillary trocars and choosing the right tools can decrease the overall hospitalization, without affecting the patient’s satisfaction and safety.
Discussion
Surgical highlights
LH is a widespread surgical procedure, that requires good surgical skills. Making this Decalogue we tried to standardize the technique with three laparoscopic accesses. Nowadays with the help of advanced electrosurgical instruments, a fourth laparoscopic access seems unessential for many gynaecological surgical procedures. In addition, the use of a uterine manipulator can be avoided by just pushing the uterine portio grasped with appropriate forceps.
Strengths and limitations
The strength of our approach is to perform type A LH using a minimal number of ancillary trocars without a manipulator and a second assistant without affecting the patient’s satisfaction and safety. The main limitation in performing this approach is related to some particular conditions related to the patient’s physical characteristics. A poor performance status secondary to comorbidities that could affect the positioning of the patient such as obesity, airflow obstruction or severe cardiopathy that justify an inadequate Trendelenburg tilting may require a traditional approach. Furthermore, this approach is not indicated for training physicians, but it should be performed by experienced surgeons.
Comparison with other surgical techniques and researches
According to the ACOG committee (15) the type of hysterectomy is based on anatomical condition, informed patient preference, and the surgeon’s expertise and training.
VH is the minimally invasive gold standard approach and is appropriate in women with mobile uteri not larger than 12 weeks gestational age. Laparoscopically assisted vaginal hysterectomy (LAVH) might be chouse in peculiar situations such as when adnexectomy must be performed for large cystitis. however, it has a longer operating time compared to VH. LH shows overlap outcomes to VH and is the preferable alternative treatment when VH is not indicated or not feasible. Subtotal hysterectomy, however, can be proposed for bleeding disorders or fibroid formation reducing the complexity of the surgery and therefore the complication rate. however, endo-bag morcellation or extraction by an extra laparotomic service access are methods used to remove the corpus uteri and might be a source of complication or delay in surgical or hospitalization time (16).
Recently a retrospective study on the feasibility and safety of a total LH without the use of a uterine manipulator demonstrated the feasibility and its safety as long as performed by well-trained and experienced laparoscopic surgeons (15,17,18).
Probing the utility of the manipulator, some researchers have compared different manipulators showing both positive and negative aspects of one rather than the other and vice versa (19).
Actually, although uterine manipulator seems to facilitate surgery, complications related to their use are also reported and, finally, no difference in safety and effectiveness are listed in the literature (20,21).
Implications and actions recommended
These findings require planning properly the surgery tailoring on the patient’s and surgeon’s characteristics. We recommend selecting the proper procedure considering the patient’s wishes and clinical characteristics keeping always high the safety standard.
Conclusions
Type A LH can be performed using three laparoscopic accesses without the aid of uterine manipulators. In selected patients and by experienced surgeons, it might reduce the invasiveness, the costs and the number of physicians involved, maintaining the same standard of safety.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-26/rc
Peer Review File: Available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-26/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-26/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this manuscript and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Garry R. Health economics of hysterectomy. Best Pract Res Clin Obstet Gynaecol 2005;19:451-65. [Crossref] [PubMed]
- Querleu D, Morrow CP. Classification of radical hysterectomy. Lancet Oncol 2008;9:297-303. [Crossref] [PubMed]
- Lansac J. Indications for hysterectomy. Contracept Fertil Sex 1997;25:816-25. [PubMed]
- Neis KJ, Zubke W, Römer T, et al. Indications and Route of Hysterectomy for Benign Diseases. Guideline of the DGGG, OEGGG and SGGG (S3 Level, AWMF Registry No. 015/070, April 2015). Geburtshilfe Frauenheilkd 2016;76:350-64. [Crossref] [PubMed]
- Morton M, Cheung VYT, Rosenthal DM. Total laparoscopic versus vaginal hysterectomy: a retrospective comparison. J Obstet Gynaecol Can 2008;30:1039-44. [Crossref] [PubMed]
- Sandberg EM, Twijnstra ARH, Driessen SRC, et al. Total Laparoscopic Hysterectomy Versus Vaginal Hysterectomy: A Systematic Review and Meta-Analysis. J Minim Invasive Gynecol 2017;24:206-217.e22. [Crossref] [PubMed]
- Drahonovsky J, Haakova L, Otcenasek M, et al. A prospective randomized comparison of vaginal hysterectomy, laparoscopically assisted vaginal hysterectomy, and total laparoscopic hysterectomy in women with benign uterine disease. Eur J Obstet Gynecol Reprod Biol 2010;148:172-6. [Crossref] [PubMed]
- Ramdhan RC, Loukas M, Tubbs RS. Anatomical complications of hysterectomy: A review. Clin Anat 2017;30:946-52. [Crossref] [PubMed]
- Van Eyk N, van Schalkwyk JInfectious Diseases Committee. Antibiotic prophylaxis in gynaecologic procedures. J Obstet Gynaecol Can 2012;34:382-91. [Crossref] [PubMed]
- Boese A, Wex C, Croner R, et al. Endoscopic Imaging Technology Today. Diagnostics (Basel) 2022;12:1262. [Crossref] [PubMed]
- Ahmad G, Duffy JM, Phillips K, et al. Laparoscopic entry techniques. Cochrane Database Syst Rev 2008;CD006583. [PubMed]
- Ahmad G, Baker J, Finnerty J, et al. Laparoscopic entry techniques. Cochrane Database Syst Rev 2019;1:CD006583. [PubMed]
- Scott MJ, Aggarwal G, Aitken RJ, et al. Consensus Guidelines for Perioperative Care for Emergency Laparotomy Enhanced Recovery After Surgery (ERAS(®)) Society Recommendations Part 2-Emergency Laparotomy: Intra- and Postoperative Care. World J Surg 2023;47:1850-80. [Crossref] [PubMed]
- Ferrari F, Forte S, Sbalzer N, et al. Validation of an enhanced recovery after surgery protocol in gynecologic surgery: an Italian randomized study. Am J Obstet Gynecol 2020;223:543.e1-543.e14. [Crossref] [PubMed]
- Swenson CW, Kamdar NS, Harris JA, et al. Comparison of robotic and other minimally invasive routes of hysterectomy for benign indications. Am J Obstet Gynecol 2016;215:650.e1-8. [Crossref] [PubMed]
- Casarin J, Ghezzi F, Dri M, et al. Laparoscopic subtotal hysterectomy followed by in-bag transvaginal corpus uteri morcellation and extraction: A case series. Eur J Obstet Gynecol Reprod Biol 2023;282:124-7. [Crossref] [PubMed]
- Zygouris D, Chalvatzas N, Gkoutzioulis A, et al. Total laparoscopic hysterectomy without uterine manipulator. A retrospective study of 1023 cases. Eur J Obstet Gynecol Reprod Biol 2020;253:254-8. [Crossref] [PubMed]
- Boztosun A, Atılgan R, Pala Ş, et al. A new method used in laparoscopic hysterectomy for uterine manipulation: uterine rein technique. J Obstet Gynaecol 2018;38:864-8. [Crossref] [PubMed]
- Misirlioglu S, Boza A, Urman B, et al. Clermont-Ferrand versus Vectec uterine manipulator for total laparoscopic hysterectomy. Minim Invasive Ther Allied Technol 2019;28:51-6. [Crossref] [PubMed]
- Abdel Khalek Y, Bitar R, Christoforou C, et al. Uterine manipulator in total laparoscopic hysterectomy: safety and usefulness. Updates Surg 2020;72:1247-54. [Crossref] [PubMed]
- Surgical steps of total laparoscopic hysterectomy: Part 1: Benign disease by the European Society for Gynaecological Endoscopy (ESGE)(1). Facts Views Vis Obgyn 2019;11:103-10. [PubMed]
Cite this article as: Chiofalo B, Valenti G, Madeo D, Vizza E, Ciancio F. A step-by-step decalogue for performing a simplified type A total laparoscopic hysterectomy using fewer accesses and tools. Gynecol Pelvic Med 2023;6:28.