
Acute myeloid leukemia after PARP inhibitor treatment in ovarian cancer—a case report and literature review
Highlight box
Key findings
• This case indicates secondary MDS or AML that can develop in patients of ovarian cancer treated with pamiparib.
What is known and what is new?
• PARP inhibitors are widely used in the maintenance therapy of ovarian cancer. A very small number of patients have developed secondary MDS or AML on the maintenance therapy with olaparib or niraparib.
• This is the first case to report AML developed after the use of pamiparib.
What is the implication, and what should change now?
• Long term safety monitoring will be necessary in discussing clinical risks and benefits of PARP inhibitors for patients with genetically susceptible tumors. It is also important to improve clinicians’ management of secondary AML and MDS during using of PARP inhibitors.
Introduction
The role of poly (adenosine diphosphate-ribose) polymerase (PARP) in solid tumors is well established in breast cancer (BRCA) pathogenic variant or homologous recombination-deficient (HRD) malignancy (1). PARP inhibitors (PARPi) have shown clinically significant improvement in progression-free survival in ovarian (2-6), breast (7,8), pancreatic (9), and prostate cancers (10). Therefore, the European Drug Administration and the US Food and Drug Administration (FDA) approved the clinical application of four PARPi in ovarian, breast, pancreatic, and prostate cancers between 2014 and 2019 (11). PARPi have been recommended as the first-line maintenance therapy for advanced epithelial ovarian cancer and as the maintenance therapy in relapsed ovarian cancer regardless of the initial International Federation of Gynecology and Obstetrics (FIGO) stage by the National Comprehensive Cancer Network and Chinese guidelines to be used for 2 to 3 years or until progression of the disease.
Some randomized controlled trials (RCTs) showed that the most common adverse events of PARPi were hematotoxicity, fatigue, and gastrointestinal toxicities, which usually occurred in the first 3 months of the treatment. However, a number of RCTs reported that patients exposed to PARPi developed myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML), which might be delayed adverse events of PARPi treatment (2-6).
This study aimed to report a case of a patient with recurrent ovarian cancer who achieved a partial response after treatment with pamiparib. She was diagnosed with treatment-related AML after months of pamiparib treatment. Although secondary MDS or AML had been reported earlier, clinicians were still inexperienced in the case of specific patients. Therefore, we reported this case and reviewed the current literature of PARPi treatment in ovarian cancer, and discussed the long-term safety monitoring. We present this case in accordance with the CARE reporting checklist (available at https://gpm.amegroups.org/article/view/10.21037/gpm-22-34/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 56-year-old woman was admitted to the center with a 2-month history of abdominal distension and a 2-week history of abdominal pain in September 2013 (the patient’s timeline is illustrated in Figure 1). She had a family history of esophagus and lung cancer. A physical examination showed a huge solid mass in the right adnexal area up to three fingers below the umbilical region involving the anterior wall of the rectum and left pelvic wall. The cancer antigen (CA) 125 level was 4,138.1 U/mL, and the computed tomography (CT) scan revealed a 128×101×114 mm3 cystic solid mass with obviously enhanced inhomogeneity, which extended to the pelvic diaphragm, pushing the bladder and uterus forward significantly. A nodular thickened omentum was seen, and large-volume ascites were observed in the abdominal and pelvic cavities. The right obturator area and para-aortic lymph nodes were enlarged to 12×15 and 12×21 mm2, respectively. She underwent aspiration of the ascitic fluid. Cytology showed that the tumor cells were consistent with the diagnosis of a high-grade serous ovarian adenocarcinoma. Gastroscopy and colonoscopy revealed no abnormality.
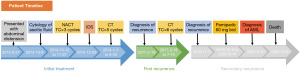
Considering primary debulking surgery could not achieve optimal cytoreduction after multidisciplinary discussion, the patient was recommended for neoadjuvant chemotherapy. The patient started a 3-week carboplatin (area under the curve: 5)/paclitaxel (175 mg/m2) regimen for three cycles. Also, she underwent a complete interval debulking surgery, and the CA 125 level reduced to 16 U/mL. She received five cycles of adjuvant chemotherapy combining carboplatin and paclitaxel. After completion of the chemotherapy, the CA 125 level was normal (5.9 U/mL), and the patient was considered having a complete response.
After 32 months of follow-up, the cancer relapsed. The recurrence was suggested based on the increase in the CA 125 level to 43.1 U/mL. In February 2017, the CT scan showed pelvic carcinomatosis around the rectum of a maximum size of 12×9 mm2. The treatment strategy was discussed with the tumor board. Secondary debulking surgery was suggested, but the patient refused because of the fear of surgical morbidity. Subsequently, she received six cycles of chemotherapy with carboplatin and paclitaxel regimen. The patient achieved a partial response to the treatment. The CA 125 level decreased to 7.8 U/mL, and the CT scan showed that pelvic carcinomatosis reduced to a size of 2 mm.
After 16 months of follow-up, the tumor relapsed (November 2018). The CA 125 level increased to 41.9 U/mL, and the CT scan revealed multiple small nodules around the rectum, with a maximum size of 12×14 mm2. Chemotherapy and surgery were still proposed; however, the patient refused to undergo the surgery again. As our center was conducting a clinical trial of PARPi for treating platinum-sensitive recurrent ovarian cancer, the patient also refused chemotherapy and agreed to the use of pamiparib, which is one of the PARPi drugs. Germline breast cancer susceptibility gene 1/2 (BRCA1/2) was analyzed using next-generation sequencing, and a pathogenic mutation was identified in BRCA2 (NM_000059.3; exon11: c.5164_5165delAG: p.S1722Yfs*4). Pamiparib taken 60 mg twice a day was administered to the patient from December 2018 to August 2020. The CA 125 level fluctuated from 3.1 to 4.4 U/mL after 3 months of pamiparib treatment. The CT scan confirmed a positive response to the treatment and showed that the maximum nodule shrank to 3×4 mm2 in August 2020. She had grade III neutropenia, anemia, and grade II thrombocytopenia during the first 2 months of pamiparib treatment. Granulocyte colony-stimulating factor (G-CSF) was used to treat neutropenia, and supportive red blood cell transfusion was given to treat anemia. During subsequent treatment with pamiparib, she continued to experience intermittent grade I–II neutropenia/anemia without any treatment.
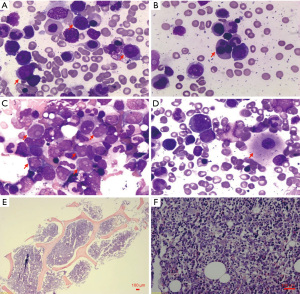
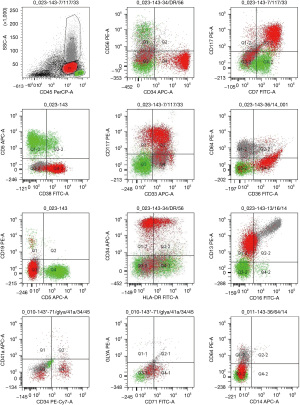
After 20 months of pamiparib treatment, the complete blood count of the patient revealed the following: neutrophil count 1.72×109/L, hemoglobin level 97 g/dL, and platelet count 64×109/L on August 5, 2020. No attention was paid to grade I neutropenia, anemia, and grade II thrombocytopenia. However, on August 26, when the patient got the blood routine test done, her neutrophil count was 1.22×109/L, hemoglobin level was 87 g/dL, and platelet count was 43×109/L. Pamiparib was discontinued, and recombinant human thrombopoietin (rhTPO) was administered to the patient. However, the patient presented with grade III–IV pancytopenia even after receiving G-CSF (rhTPO), recombinant human erythropoietin, and blood transfusion for 1 month. It was only then that we discovered the patient might have a secondary blood system disease. She was suggested to get a bone marrow smear done. It revealed obvious hyperplasia of nucleated cells with an increased number of myeloblasts and marked dysplasia (Figure 2A-2D). The bone marrow biopsy section showed prominent hypercellularity (Figure 2E) with hyperplasia of blast cells (Figure 2F) and a marked decrease in megakaryopoiesis. The concurrent flow cytometric analysis detected 25% phenotypically abnormal myeloid precursors that expressed CD34, CD13, CD33, CD36, and CD117 (Figure 3). The morphologic features and flow cytometric analysis of her bone marrow supported the diagnosis of t-AML according to the World Health Organization classification of tumors of hematopoietic and lymphoid tissues. The patient was treated with symptomatic therapy after diagnosis due to repeated pancytopenia. After 2 months, she died due to hematemesis at home.
Discussion
Olaparib was the first licensed PARPi. The FDA approved olaparib for treating BRCA-mutated ovarian cancer in patients who received three or more chemotherapy lines in December 2014. Then, in December 2018, olaparib was approved as the frontline maintenance therapy in patients with advanced ovarian cancer with harmful or suspected harmful germline or somatic BRCA mutations who achieved complete or partial response to first-line platinum-based chemotherapy. Subsequently, in October 2019, niraparib was approved in the United States for treating patients with HRD advanced ovarian cancer pre-treated with three or more prior chemotherapy lines. Gradually, PARPi have become the maintenance treatment for extending the interval of chemotherapy in ovarian cancer. Table 1 shows the clinical characteristics of reported PARPi-related RCTs on ovarian cancer (2-6,12-18). These RCTs of ovarian cancer involved four PARPi, which were used in all patients or patients with BRCA mutations as frontline maintenance or second-line or multiline therapy. Due to the widespread use of PARPi and the incidence of MDS and AML, 0.73% of patients have started using PARPi (19). We should raise awareness of the potential risk of serious adverse reactions such as MDS/AML.
Table 1
| Author | Median age (years) | Patient groups | PARPi | Front-line maintenance or for recurrent cancer | Treatment group | Control group | MDS or AML events | Previous lines of chemotherapy | Median follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| Coleman et al. 2017 (2) | 61 (PARPi) vs. 62 (control) | All patients | Rucaparib | Recurrent | Rucaparib 600 mg bid (n=372) | Placebo (n=189) | 3 (PARPi) vs. 0 (control) |
Two | Ongoing (>24.0) |
| Pujade-Lauraine et al. 2017 (3) | 56 (PARPi) vs. 56 (control) | Patients with BRCA mutations | Olaparib | Recurrent | Olaparib 300 mg bid (n=195) | Placebo (n=99) | 2 (PARPi) vs. 0 (control) |
Two | 66.0 (PARPi) vs. 64.8 (control) |
| Moore et al. 2018 (4) | 53 (PARPi) vs. 53 (control) | Patients with BRCA mutations | Olaparib | Front-line | Olaparib 300 mg bid (n=260) | Placebo (n=131) | 3 (PARPi) vs. 0 (control) |
One | 57.6 (PARPi) vs. 60.0 (control) |
| González-Martín et al. 2019 (5) | 62 (PARPi) vs. 62 (control) | All patients | Niraparib | Front-line | Niraparib 300 mg qd (n=484) | Placebo (n=244) | 1 (PARPi) vs. 1 (control) |
One | Ongoing (>24.0) |
| Penson et al. 2020 (6) | 59 (PARPi) vs. 60 (control) | Patients with BRCA mutations | Olaparib | Recurrent | Olaparib 300 mg bid (n=178) | Single chemotherapy (n=88) | 3 (PARPi) vs. 1 (control) |
Two | N/A |
| Ledermann et al. 2012 (12) | 58 (PARPi) vs. 59 (control) | All patients | Olaparib | Recurrent | Olaparib 400 mg bid (n=136) | Placebo (n=128) | 1 (PARPi) vs. 0 (control) |
Two | 78.0 |
| Mirza et al. 2016 (13) | 57 (gBRCAm PARPi) vs. 58 (gBRCAm placebo) and 63 (non-gBRCAm PARPi) vs. 61 (non-gBRCAm placebo) | All patients | Niraparib | Recurrent | Niraparib 300 mg qd (n=367) | Placebo (n=179) | 2 (PARPi) vs. 0 (control) |
Two | 24.0 |
| Coleman et al. 2019 (14) | 62 (PARPi followed by placebo) vs. 62 (PARPi) vs. 62 (placebo) | All patients | Veliparib | Front-line | TC plus veliparib followed by placebo maintenance (n=383) or TC plus veliparib followed by veliparib maintenance (n=382) | TC plus placebo followed by placebo maintenance (n=375) | 2 (PARPi) vs. 0 (control) |
Concomitantly or after first-line chemotherapy | 28.0 |
| Ray-Coquard et al. 2019 (15) | 61 (PARPi) vs. 60 (control) | All patients | Olaparib | Front-line | Olaparib 300 mg bid plus bevacizumab (n=535) | Placebo plus bevacizumab (n=267) | 7 (PARPi) vs. 2 (control) |
One | 35.5 (PARPi) vs. 36.5 (control) |
| Oza et al. 2015 (16) | 59 (PARPi) vs. 62 (control) | All patients | Olaparib | Recurrent | TC plus olaparib 200 mg bid (n=81) | TC (n=75) | 1 (PARPi) vs. 0 (control) |
One to three | 33.4 (PARPi) vs. 32.2 (control) |
| Kaye et al. 2012 (17) | 57 (PARPi 200 mg) vs. 53 (PARPi 400 mg) vs. 54 (control) | Patients with BRCA mutations | Olaparib | Recurrent | Olaparib 200 mg bid (n=32) or olaparib 400 mg bid (n=32) | Liposomal doxorubicine (n=32) | 1 (PARPi) vs. 0 (control) |
One to five | N/A |
| Kummar et al. 2015 (18) | 58 | All patients | Veliparib | Recurrent | Veliparib 60 mg + cyclophosphamide (n=37) | Cyclophosphamide (n=38) | 0 (PARPi) vs. 0 (control) |
One to nine | N/A |
PARPi, poly (adenosine diphosphate-ribose) polymerase inhibitor; MDS, myelodysplastic syndrome; AML, acute myeloid leukemia; BRCA, breast cancer; N/A, not applicable; gBRCAm, germline BRCA mutations; TC, paclitaxel and carboplatin.
PARPs are a superfamily of 18 multifunctional enzymes that play a key role in cell differentiation, transformation, and DNA single-strand break repair. The enzymes bind to DNA single-strand breaks and activate the base excision repair pathway. PARPi target the PARP family and turn single-strand breaks into double-strand breaks, which are usually repaired by homologous recombination (HR). In the way of synthetic lethality, tumor cell death occurs in HRD cells in response to PARPi, while the normal cells do not develop any solitary mutation. When lacking HR deficiency, as in BRCA-mutant cells, DNA double-strand breaks will be processed by alternative but error-prone repair pathway—non-homologous end joining repair (NHEJ)—which lead to the accumulation of genomic instability and ultimately cancer cell death. NHEJ is faster than HR. Beyond the already-known proteins, such as Ku70/80, DNA-PKcs, Artemis, DNA pol λ/µ, DNA ligase IV-XRCC4, and XLF, new proteins are involved in the NHEJ, namely PAXX, MRI/CYREN, TARDBP of TDP-43, IFFO1, ERCC6L2, and RNase H2. Among them, MRI/CYREN has dual role, as it stimulates NHEJ in the G1 phase of the cell cycle, while it inhibits the pathway in the S and G2 phases (20). A study suggested that cancer therapy, such as radiotherapy and platinum-based chemotherapy, preferentially involved mutations in genes related to DNA damage response (DDR), which shaped the fitness landscape of clonal hematopoiesis (21). However, for patients with BRCA wild-type tumors and platinum-resistant disease, PARP inhibitors exhibit very low activity as monotherapy. The combinations of PARP inhibitors with drugs that inhibit HR may sensitise ovarian cancer with a primary or secondary HR proficiency to PARP inhibitors and potentially expand their use beyond HR-deficient ovarian cancers. Regarding this, PARP inhibitors may be combined separately with anti-angiogenics and immune checkpoint inhibitors as well as with PI3K, AKT, mTOR, WEE1, MEK, and CDK4/6 inhibitors, or even with standard chemotherapy (22). Meanwhile, the DDR-mutated clonal hematopoiesis could be a risk for MDS and AML (19). Moreover, PARP is a vital part of the DDR. Another study showed that patients with Fanconi anemia with both partner and localizer of breast cancer 2 (PALB2) and BRCA2 allele mutations have an 800-fold increased risk of developing MDS or AML (23). Therefore, our patient had germline BRCA2 gene mutation, which might be one of the reasons why she was sensitive to PARPi therapy but also had increased susceptibility to AML.
Pamiparib, a selective inhibitor of PARP1 and PARP2, was developed and approved in China for treating recurrent ovarian, fallopian tube, and peritoneal cancers. An X-ray eutectic structure showed that the drug bound to similar sites of olaparib and niraparib and exerted antitumor effects. However, different from olaparib and niraparib, pamiparib is not the substrate of p-glycoprotein (P-gp), which is overexpressed in tumor cells and associated with a variety of antitumor drug resistance. Therefore, pamiparib can avoid the resistance caused by the upregulation of P-gp gene expression and P-gp substrate drugs (24). The PARP-DNA complex can be captured by pamiparib at a very low drug concentration (25). At the same time, pamiparib has better solubility and permeability than olaparib; only one-sixteenth pamiparib achieves similar antitumor efficacy as olaparib in vivo. Technologies of proteomics, such asproteomics analysis of ovarian cancer, as well as their adaptive responses to therapy, can uncover new therapeutic choices, which can reduce the emergence of drug resistance and potentially improve patient outcomes (26).
However, whether the secondary AML of the patient in this study was caused by PARPi has not been confirmed. The patient had received two-line platinum-based chemotherapy prior to PARPi use, and the chemotherapy might also develop secondary cancer. Recently, two meta-analyses discussed whether PARPi caused MDS or AML, and the conclusions were not completely consistent. Based on 18 placebo RCTs, Morice et al. showed that the administration of PARPi significantly increased the risk of MDS or AML in patients with ovarian, breast, pancreatic, and prostate cancers (19). However, Nitecki et al. found that the risk of MDS or AML was similar in patients who received PARPi compared with controls by analyzing 14 published studies on PARPi (27). The author considered that the different results might be due to different statistical methods. However, in a subgroup analysis, Nitecki et al. showed that PARPi treatment increased the risk of MDS or AML in patients on frontline maintenance therapy, but no such association was found in patients who had relapsed the disease. Furthermore, PARPi use was associated with an increased incidence of MDS or AML in biomarker-unrestricted patients, while the risk of MDS or AML was not increased in patients with BRCA mutation groups (27). The data suggested that the statistical difference in MDS or AML caused by PARPi was not reflected in patients with recurrent disease who received prior chemotherapy. Patients with BRCA mutations were susceptible to MDS or AML; therefore, MDS or AML caused by PARPi was not seen in the BRCA mutation group, but in the whole population.
Morice et al. analyzed the cases from the VigiBase database and found that MDS or AML occurred after using PARPi with a median latency period of 17.8 months (19). MDS occurred 17.8 months after the first exposure to PARPi. AML occurred 20.6 months after initial exposure to PARPi. The patient in this study developed AML after 20 months of PARPi exposure, consistent with previous studies. However, when the blood routine showed a platelet count of 64×109/L, which remained steady above 100×109/L for the previous year, PARPi treatment was not stopped. When pancytopenia occurred in the patient, symptomatic treatment was found to be ineffective. Therefore, every change in the blood routine should be monitored, especially a sudden decrease in the blood cell count, which remains normal for a long time. At the same time, since the majority of secondary MDS or AML occurred after 1 year of PARPi use, it is also important to monitor patients during PARPi therapy. Therefore, the long-term application of PARPi should be done with great caution.
It is provided a case with secondary AML developed after the treatment using a PARP inhibitor following platinum-sensitive recurrence in detail, hoping to improve clinicians’ understanding intuitively. However, the study also has some limitations. Such as the case report only provided limited information of one patient. Moreover, the patient died soon after diagnosis of AML, and no treatment strategy for secondary AML was provided. We reviewed the clinical characteristics of reported PARPi-related RCTs on ovarian cancer, hoping to make up for the lacks of case report. The studies provide the incidence of secondary MDS/AML after use of PARPi, the median latency period MDS/AML occurred after using PARPi et al. These data provided clinicians a more comprehensive understanding of secondary MDS/AML after use of PARPi.
Conclusions
In summary, the present study described the development of secondary AML after second-line treatment using a PARPi in a patient with ovarian cancer with germline BRCA2 gene mutation following platinum-sensitive recurrence. As PARPi are widely used in ovarian cancer, it is important to evaluate the long-term safety and pay attention to fatal complications. Further clinical studies are expected to focus on the safe long-term use of PARPi.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (No. 81902649), the Key Project of Sichuan Provincial Department of Science and Technology (No. 2019YFS0532), and the Project of Chengdu Science and Technology Administration (No. 2021-YF05-01725-SN).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://gpm.amegroups.org/article/view/10.21037/gpm-22-34/rc
Peer Review File: Available at https://gpm.amegroups.org/article/view/10.21037/gpm-22-34/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm-22-34/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fritz C, Portwood SM, Przespolewski A, et al. PARP goes the weasel! Emerging role of PARP inhibitors in acute leukemias. Blood Rev 2021;45:100696. [Crossref] [PubMed]
- Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:1949-61. [Crossref] [PubMed]
- Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1274-84. [Crossref] [PubMed]
- Moore K, Colombo N, Scambia G, et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 2018;379:2495-505. [Crossref] [PubMed]
- González-Martín A, Pothuri B, Vergote I, et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 2019;381:2391-402. [Crossref] [PubMed]
- Penson RT, Valencia RV, Cibula D, et al. Olaparib Versus Nonplatinum Chemotherapy in Patients With Platinum-Sensitive Relapsed Ovarian Cancer and a Germline BRCA1/2 Mutation (SOLO3): A Randomized Phase III Trial. J Clin Oncol 2020;38:1164-74. [Crossref] [PubMed]
- Robson M, Im SA, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med 2017;377:523-33. [Crossref] [PubMed]
- Litton JK, Rugo HS, Ettl J, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med 2018;379:753-63. [Crossref] [PubMed]
- Golan T, Hammel P, Reni M, et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med 2019;381:317-27. [Crossref] [PubMed]
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020;382:2091-102. [Crossref] [PubMed]
- Mateo J, Lord CJ, Serra V, et al. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol 2019;30:1437-47. [Crossref] [PubMed]
- Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 2012;366:1382-92. [Crossref] [PubMed]
- Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med 2016;375:2154-64. [Crossref] [PubMed]
- Coleman RL, Fleming GF, Brady MF, et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N Engl J Med 2019;381:2403-15. [Crossref] [PubMed]
- Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med 2019;381:2416-28. [Crossref] [PubMed]
- Oza AM, Cibula D, Benzaquen AO, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol 2015;16:87-97. [Crossref] [PubMed]
- Kaye SB, Lubinski J, Matulonis U, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol 2012;30:372-9. [Crossref] [PubMed]
- Kummar S, Oza AM, Fleming GF, et al. Randomized Trial of Oral Cyclophosphamide and Veliparib in High-Grade Serous Ovarian, Primary Peritoneal, or Fallopian Tube Cancers, or BRCA-Mutant Ovarian Cancer. Clin Cancer Res 2015;21:1574-82. [Crossref] [PubMed]
- Morice PM, Leary A, Dolladille C, et al. Myelodysplastic syndrome and acute myeloid leukaemia in patients treated with PARP inhibitors: a safety meta-analysis of randomised controlled trials and a retrospective study of the WHO pharmacovigilance database. Lancet Haematol 2021;8:e122-34. [Crossref] [PubMed]
- Boussios S, Rassy E, Moschetta M, et al. BRCA Mutations in Ovarian and Prostate Cancer: Bench to Bedside. Cancers (Basel) 2022;14:3888. [Crossref] [PubMed]
- Bolton KL, Ptashkin RN, Gao T, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet 2020;52:1219-26. [Crossref] [PubMed]
- Shah S, Cheung A, Kutka M, et al. Epithelial Ovarian Cancer: Providing Evidence of Predisposition Genes. Int J Environ Res Public Health 2022;19:8113. [Crossref] [PubMed]
- Churpek JE, Marquez R, Neistadt B, et al. Inherited mutations in cancer susceptibility genes are common among survivors of breast cancer who develop therapy-related leukemia. Cancer 2016;122:304-11. [Crossref] [PubMed]
- Xiong Y, Guo Y, Liu Y, et al. Pamiparib is a potent and selective PARP inhibitor with unique potential for the treatment of brain tumor. Neoplasia 2020;22:431-40. [Crossref] [PubMed]
- Wang H, Ren B, Liu Y, et al. Discovery of Pamiparib (BGB-290), a Potent and Selective Poly (ADP-ribose) Polymerase (PARP) Inhibitor in Clinical Development. J Med Chem 2020;63:15541-63. [Crossref] [PubMed]
- Ghose A, Gullapalli SVN, Chohan N, et al. Applications of Proteomics in Ovarian Cancer: Dawn of a New Era. Proteomes 2022;10:16. [Crossref] [PubMed]
- Nitecki R, Melamed A, Gockley AA, et al. Incidence of myelodysplastic syndrome and acute myeloid leukemia in patients receiving poly-ADP ribose polymerase inhibitors for the treatment of solid tumors: A meta-analysis of randomized trials. Gynecol Oncol 2021;161:653-9. [Crossref] [PubMed]
Cite this article as: Zeng J, Wu J, Li Q, Li K, Wang D, Yin R. Acute myeloid leukemia after PARP inhibitor treatment in ovarian cancer—a case report and literature review. Gynecol Pelvic Med 2023;6:18.


