
A survey of gynecologists’ utilization of tranexamic acid and factors influencing prophylactic use of tranexamic acid
Introduction
In gynecology, operative bleeding during surgical procedures as well as bleeding from benign gynecologic conditions are leading causes of morbidity for women (1,2). The anti-fibrinolytic agent, tranexamic acid (TXA), has been shown to decrease blood loss throughout a variety of specialties and may be an adjunct that can be useful in gynecologic surgery. TXA is a relatively safe, low-cost, and easy to administer medication. It is a synthetic lysine derivative that reversibly binds to plasminogen, essentially stabilizing the clot and inhibiting fibrinolysis to reduce bleeding (3).
TXA has been utilized in gynecology since the 1970s and was first described in a trial published in the British Medical Journal showing that TXA decreased menstrual blood loss in women with menorrhagia (4). The benefits of TXA in reducing bleeding complications is well-documented in the trauma, orthopedic, cardiac and obstetric literature, showing that TXA is associated with decrease in acute blood loss, blood transfusion, and death (5-8).
In recent years, randomized control trials have investigated TXA use in gynecology, which includes the use for heavy menstrual bleeding (HMB) and during benign hysterectomy and myomectomy (9-12). TXA has been well-studied in the literature as a non-hormonal medication to reduce HMB and has been found to decrease HMB by 40% compared to placebo (12). In a trial of women undergoing myomectomy, prophylactic TXA use was associated with a reduction of intraoperative blood loss (10). A systematic review evaluating the effect of TXA in gynecologic and obstetric surgeries showed a reduction in blood loss of 26% compared to controls (13). In a recent randomized trial of women undergoing hysterectomy, prophylactic TXA significantly lowered total blood loss and the need for reoperations due to postoperative hemorrhage (11).
The above studies have demonstrated the potential effectiveness of TXA in reducing surgical bleeding during hysterectomy and myomectomy procedures. Despite this data, the use of TXA for benign gynecologic surgery is not routine preoperative practice and may be underutilized. The use of TXA has been widely incorporated into obstetric protocols and is recommended by the World Health Organization (WHO) for the treatment of postpartum hemorrhage (14). In contrast, current perioperative guidelines in gynecologic surgery do not include recommendations for or against the use of TXA as a preoperative or intraoperative adjunct. Furthermore, no studies to date have been published investigating the factors influencing the use of TXA by gynecologists. The objectives of our study are to determine current practice patterns and utilization of TXA by gynecologists and to examine the factors influencing the prophylactic use of TXA by surveying members of AAGL (formerly known as the American Association of Gynecologic Laparoscopists). We present this article in accordance with the SURGE reporting checklist (available at https://gpm.amegroups.com/article/view/10.21037/gpm-22-12/rc).
Methods
Sampling and subjects
AAGL members were invited via e-mail to participate in an Internet-based survey on TXA use in gynecology. AAGL is a global organization representing gynecologists from 110 countries, whose focus is promoting minimally invasive gynecologic surgery among surgeons worldwide. The survey was approved by the AAGL Research Committee and AAGL Board of Directors. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The survey received exempt status from the Loyola University Chicago Health Sciences Division Institutional Review Board (IRB #211981). Informed consent was not required since the research involved no more than minimal risk to subjects.
Instruments
The survey collected basic demographic information including age, sex, type of practice, number of years in practice, fellowship training, and number of total gynecologic cases performed per year. Respondents were asked about oral TXA prescribing practices for cyclic HMB and use of TXA for acute bleeding during gynecologic surgery. Additional survey questions included selective versus routine use of prophylactic TXA during myomectomy and hysterectomy, preferred dosage of prophylactic TXA, comfort level with TXA use, and factors influencing use of TXA. Concerns about using TXA and perceived barriers to TXA use were also assessed. The questions and variables are listed in Table 1.
Table 1
| Primary practice location |
| Gender |
| Age |
| Years in practice |
| Practice setting |
| Completion of fellowship training |
| Type of fellowship completed |
| Total number of gynecologic surgeries performed in 1 year |
| Do you prescribe oral TXA for patients with cyclic heavy menstrual bleeding? |
| Are you familiar with the CRASH-2 trial (a randomized controlled trial on the effects of TXA in adult trauma patients, Lancet 2010)? |
| Have you ever used TXA to treat acute bleeding in a patient in a gynecological surgical setting? |
| How do you describe your clinical use of TXA in gynecologic surgery? |
| What percentage of the time do you use prophylactic TXA for myomectomy? |
| What percentage of the time do you use prophylactic TXA for hysterectomy? |
| What prophylactic dosage of TXA do you use for gynecologic surgery? |
| How comfortable are you with using TXA during gynecologic surgery? |
| What are you concerns with use of TXA? |
| Factors influencing decision to administer prophylactic TXA |
| Surgeries in which you have used prophylactic TXA |
TXA, tranexamic acid.
Procedures
The survey was initially sent electronically to the mailing list of 5,764 AAGL members in July 2019. A second and final reminder for survey completion was included in the electronic membership newsletter distributed in August 2019.
Statistical analysis
The survey was sent to all AAGL members and was analyzed based on the total number of respondents. No a-priori sample size justification is needed since the entire census of individuals were included in the potential population of interest. Data were reported descriptively. All surveys were completed in their entirety. Differences between categorical variables were assessed using Chi-square tests. Two-sided P values of <0.05 were considered statistically significant. All analyses were performed with SAS 9.4 (Cary, NC).
Results
A total of 314 AAGL members responded to the survey (response rate of 5.4%) and all were included in the final analysis. The demographic characteristics of respondents are listed in Table 2. The majority of participants were affiliated with an academic medical center (49.0%). Fifty percent of respondents had completed fellowship training with the majority of fellowship-trained physicians having completed a minimally invasive gynecologic surgery fellowship (71.8%). Thirty-seven percent of respondents reported being in practice for >20 years and 63.4% reported being in practice for 20 years or less. The vast majority of respondents performed >100 surgeries per year (65.3%).
Table 2
| Demographics | n (%) |
|---|---|
| Location of primary practice | |
| In the United States | 183 (58.3) |
| Outside of the United States | 131 (41.7) |
| Gender | |
| Female | 145 (46.2) |
| Male | 169 (53.8) |
| Age (years) | |
| 26–35 | 69 (22.0) |
| 36–45 | 94 (29.9) |
| 46–55 | 59 (18.8) |
| 56–65 | 59 (18.8) |
| >65 | 33 (10.5) |
| Years in practice | |
| 1–5 years | 97 (30.9) |
| 6–10 years | 54 (17.2) |
| 11–20 years | 48 (15.3) |
| >20 years | 115 (36.6) |
| Practice settinga | |
| Academic medical center | 154 (49.0) |
| Community-based hospital | 88 (28.0) |
| Private practice | 128 (40.8) |
| Government/military | 8 (2.6) |
| Other | 2 (0.6) |
| Fellowship training | |
| None | 138 (43.9) |
| Completed | 156 (49.7) |
| Currently in fellowship | 20 (6.4) |
| Type of fellowship completed | |
| Female pelvic medicine and reconstructive surgery | 12 (7.7) |
| Gynecologic oncology | 8 (5.1) |
| Minimally invasive gynecologic surgery | 112 (71.8) |
| Reproductive endocrinology and infertility | 13 (8.3) |
| Other | 11 (7.1) |
| Total number of gynecologic surgeries performed in one year | |
| 0–100 | 108 (34.4) |
| 101–200 | 98 (31.2) |
| 201–300 | 71 (22.6) |
| >300 | 36 (11.5) |
| Unknown | 1 (0.32) |
a, greater than 100% due to multiple choices selected.
Eighty-four percent of providers (n=263) reported having prescribed oral TXA for treatment of cyclic HMB. Table 3 summarizes the oral use TXA practice pattern and provider characteristics. There were no significant differences in oral use TXA practice patterns by any of the provider characteristics including provider age, number of years in practice, fellowship training, surgical volume or practice setting.
Table 3
| Characteristics | All (n=314) | Oral use | P value* | |
|---|---|---|---|---|
| No (n=51) | Yes (n=263) | |||
| Age (years) | 0.086 | |||
| 26–35 | 69 [22] | 10 [20] | 59 [22] | |
| 36–45 | 94 [30] | 12 [24] | 82 [31] | |
| 46–55 | 59 [19] | 9 [18] | 50 [19] | |
| 56–65 | 59 [19] | 9 [18] | 50 [19] | |
| >65 | 33 [11] | 11 [22] | 22 [8] | |
| Years in practice | 0.197 | |||
| 1–5 years | 97 [31] | 12 [24] | 85 [32] | |
| 6–10 years | 54 [17] | 6 [12] | 48 [18] | |
| 11–20 years | 48 [15] | 8 [16] | 40 [15] | |
| >20 years | 115 [37] | 25 [49] | 90 [34] | |
| Fellowship training | 0.391 | |||
| No fellowship or current fellowship | 138 [44] | 26 [51] | 112 [43] | |
| Minimally invasive gynecologic surgery fellowship | 112 [36] | 14 [27] | 98 [37] | |
| Other fellowship | 64 [20] | 11 [22] | 53 [20] | |
| Total number of gynecologic surgeries performed in one year | 0.998 | |||
| 0–100 | 108 [35] | 17 [34] | 91 [35] | |
| 101–200 | 98 [31] | 16 [32] | 82 [31] | |
| 201–300 | 71 [23] | 11 [22] | 60 [23] | |
| >300 | 36 [12] | 6 [12] | 30 [11] | |
| Practice setting | 0.088 | |||
| Academic medical center | 114 [36] | 12 [24] | 102 [39] | |
| Community-based hospital | 58 [18] | 8 [16] | 50 [19] | |
| Private practice | 77 [25] | 18 [35] | 59 [22] | |
| Other/multiple institutions | 65 [21] | 13 [25] | 52 [20] | |
Data are presented as n [%]. *, P value calculated by Chi-square test.
Overall, 58.6% of respondents (n=184) reported use of TXA to treat acute bleeding in a patient in a gynecologic surgical setting (Table 4). Providers affiliated with a private practice setting were less likely to use TXA for acute bleeding compared to those affiliated with an academic medical center (19% vs. 36%, P=0.023). Acute use of TXA did not differ by provider age, number of years in practice, fellowship training surgical volume.
Table 4
| Characteristics | All (n=314) | Acute use | P value* | |
|---|---|---|---|---|
| No (n=130) | Yes (n=184) | |||
| Age (years) | 0.088 | |||
| 26–35 | 69 [22] | 32 [25] | 37 [20] | |
| 36–45 | 94 [30] | 28 [22] | 66 [36] | |
| 46–55 | 59 [19] | 29 [22] | 30 [16] | |
| 56–65 | 59 [19] | 25 [19] | 34 [18] | |
| >65 | 33 [11] | 16 [12] | 17 [9] | |
| Years in practice | 0.085 | |||
| 1–5 years | 97 [31] | 38 [29] | 59 [32] | |
| 6–10 years | 54 [17] | 15 [12] | 39 [21] | |
| 11–20 years | 48 [15] | 23 [18] | 25 [14] | |
| >20 years | 115 [37] | 54 [42] | 61 [33] | |
| Fellowship training | 0.701 | |||
| No fellowship or current fellowship | 138 [44] | 54 [42] | 84 [46] | |
| Minimally invasive gynecologic surgery fellowship | 112 [36] | 47 [36] | 65 [35] | |
| Other fellowship | 64 [20] | 29 [22] | 35 [19] | |
| Total number of gynecologic surgeries performed in one year | 0.377 | |||
| 0–100 | 108 [35] | 43 [33] | 65 [35] | |
| 101–200 | 98 [31] | 41 [32] | 57 [31] | |
| 201–300 | 71 [23] | 34 [26] | 37 [20] | |
| >300 | 36 [12] | 11 [9] | 25 [14] | |
| Practice setting | 0.023 | |||
| Academic medical center | 114 [36] | 48 [37] | 66 [36] | |
| Community-based hospital | 58 [18] | 19 [15] | 39 [21] | |
| Private practice | 77 [25] | 42 [32] | 35 [19] | |
| Other/multiple institutions | 65 [21] | 21 [16] | 44 [24] | |
Data are presented as n [%]. *, P value calculated by Chi-square test.
The majority of respondents did not use prophylactic TXA for gynecologic surgery (57.3%, n=180). Surgeons who completed a minimally invasive gynecologic surgery fellowship were more likely to use TXA for prophylaxis (Table 5). Of surgeons using TXA in a prophylactic manner (n=134), 91% used TXA selectively whereas 9% used TXA for all gynecologic surgical cases. Among surgeons using prophylactic TXA, use was most common for laparoscopic or robotic myomectomy procedures (31.9%) and open myomectomy procedures (30.2%). Prophylactic TXA was less frequently utilized for hysterectomy procedures (15.9% laparoscopic or robotic; 6.4% vaginal; 13.7% open hysterectomy). The most common dosage administered was a single dose of 1 gram TXA prior to surgery.
Table 5
| Characteristics | All (n=314) | Prophylactic use | P value* | |
|---|---|---|---|---|
| No (n=180) | Yes (n=134) | |||
| Age (years) | 0.146 | |||
| 26–35 | 69 [22] | 35 [19] | 34 [25] | |
| 36–45 | 94 [30] | 50 [28] | 44 [33] | |
| 46–55 | 59 [19] | 40 [22] | 19 [14] | |
| 56–65 | 59 [19] | 32 [18] | 27 [20] | |
| >65 | 33 [11] | 23 [13] | 10 [7] | |
| Years in practice | 0.317 | |||
| 1–5 years | 97 [31] | 49 [27] | 48 [36] | |
| 6–10 years | 54 [17] | 30 [17] | 24 [18] | |
| 11–20 years | 48 [15] | 31 [17] | 17 [13] | |
| >20 years | 115 [37] | 70 [39] | 45 [34] | |
| Fellowship training | 0.006 | |||
| No fellowship or current fellowship | 138 [44] | 92 [51] | 46 [34] | |
| Minimally invasive gynecologic surgery fellowship | 112 [36] | 52 [29] | 60 [45] | |
| Other fellowship | 64 [20] | 36 [20] | 28 [21] | |
| Total number of gynecologic surgeries performed in one year | 0.640 | |||
| 0–100 | 108 [35] | 63 [35] | 45 [34] | |
| 101–200 | 98 [31] | 58 [32] | 40 [30] | |
| 201–300 | 71 [23] | 41 [23] | 30 [22] | |
| >300 | 36 [12] | 17 [9] | 19 [14] | |
| Practice setting | 0.187 | |||
| Academic medical center | 114 [36] | 57 [32] | 57 [43] | |
| Community-based hospital | 58 [18] | 33 [18] | 25 [19] | |
| Private practice | 77 [25] | 50 [28] | 27 [20] | |
| Other/multiple institutions | 65 [21] | 40 [22] | 25 [19] | |
Data are presented as n [%]. *, P value calculated by Chi-square test.
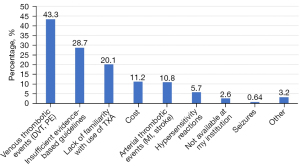
Most respondents were either somewhat or very comfortable (29.6%, n=93 and 51.9%, n=163) with using TXA during gynecologic surgery. The most common factors influencing the decision to administer prophylactic TXA were uterine/myoma size (91.0%) and preoperative hemoglobin level (72.4%). Duration and route of surgery were less influential factors in determination of prophylactic TXA use. The most common provider concerns for using TXA were venous thrombotic events (43.3%) and insufficient evidence-based guidelines (28.7%) (Figure 1). Less frequently cited concerns included lack of familiarity with TXA use, cost, arterial thrombotic events and hypersensitivity reactions. The greatest perceived barriers to TXA use in gynecologic surgery were lack of surgeon knowledge/familiarity with TXA use (71.0%) and insufficient evidence regarding efficacy and safety (41.1%) (Figure 2).
Discussion
The utilization of TXA has been well-described in gynecology, ranging from its use as a non-hormonal treatment of HMB or acute/prolonged uterine bleeding as well as an intraoperative adjunct for the reduction of surgical bleeding during myomectomy or hysterectomy. In our survey study of AAGL members, most respondents reported having prescribed oral TXA for the treatment of HMB. The use of TXA was less frequent for acute bleeding in gynecologic surgery but was reported by over half of the respondents. The least frequent indication for TXA use reported among respondents was prophylactic use at the time of myomectomy or hysterectomy. When used prophylactically, the majority of gynecologic surgeons used TXA in a selective manner based upon uterine/myoma size and preoperative hemoglobin level. Our survey also demonstrated that significant variations in practice patterns exist for the use of TXA depending on fellowship training status and practice setting.
There are numerous studies establishing the utility and efficacy of TXA within gynecology. For women with HMB, greater reduction in menstrual blood loss can be achieved by TXA in comparison to placebo or NSAIDs alone (12,15). TXA has not been specifically studied for the treatment of acute AUB but based upon expert opinion, has been suggested as a potential therapeutic regimen (16,17). Recently, TXA has been evaluated in randomized controlled trials as an intraoperative adjunct to reduce blood loss during gynecologic surgery. In women undergoing benign hysterectomy, prophylactic intravenous TXA significantly reduced overall total blood loss and substantial blood loss (≥500 mL) as well as the need for reoperation compared to placebo (11). Similar results were demonstrated in another RCT showing greater reduction in intraoperative and postoperative blood loss during abdominal hysterectomy in groups receiving intravenous or topical TXA compared to placebo (18). Randomized trials evaluating the use of TXA for myomectomy have shown conflicting findings. In one study of women undergoing abdominal myomectomy, patients receiving a 10 mg/kg dose of TXA followed by continuous infusion of TXA had significantly decreased blood loss and higher postoperative hemoglobin levels (10). However, in three RCTs of abdominal myomectomy, intravenous TXA did not significantly reduce perioperative blood loss compared to placebo (19-21).
The strategies used by gynecologic surgeons to reduce perioperative blood loss are extensive, ranging from preoperative anemia optimization to intraoperative hemostasis techniques. The use of specific agents and interventions may vary considerably from surgeon to surgeon. Surgical hemostasis can be accomplished through mechanical ligation with suture or clips, use of electrosurgery, or application of topical hemostatic agents. TXA can be a useful pharmacologic adjunct to reduce blood loss during surgery due to its antifibrinolytic properties. The activation of fibrinolysis is a critical step that leads to intraoperative and postoperative blood loss. TXA is a synthetic lysine analogue that inhibits conversion of plasminogen to plasmin, thereby preventing fibrin cleavage and promoting hemostasis (22,23). Only one other study in the literature has assessed the usage of perioperative agents by obstetrician-gynecologists for reducing blood loss during gynecologic surgery (24). In this survey study of Royal College of Physicians and Surgeons of Canada, multiple medications and interventions were evaluated; 73.5% of respondents reported use of intravenous TXA during myomectomy. Our survey study differs due to the specific focus on the use of TXA in gynecology on a broader scale including, but not limited to myomectomy. In contrast to the Canadian survey, only 43% of our survey respondents utilized TXA in a prophylactic manner, though the use of prophylactic TXA was more frequent for myomectomy cases compared to hysterectomy cases.
The cost-effectiveness of TXA administration to reduce bleeding complications in the obstetric and trauma population has been established in several landmark studies, the WOMAN and CRASH-2 trials, respectively. Although the cost-effectiveness has not been directly evaluated for patients undergoing gynecologic surgery, TXA is considered to be an inexpensive medication with costs (per 1 gram dose in US$) ranging from $5-6 (Pakistan/UK/India/Tanzania) to $29.84 (Nigeria) (25,26). In our study, the cost of TXA was reported as a potential concern in only 11.2% of respondents.
In our study, the use of TXA for acute bleeding and prophylactic measures was less common compared to respondents’ use of TXA for treatment of chronic abnormal uterine bleeding. This practice pattern of TXA utilization may be multifactorial in nature. We sought to examine the potential concerns and barriers regarding TXA use among respondents. Throughout the literature, a commonly cited potential adverse effect of TXA is the risk of thromboembolic events. Notably, the leading concern reported by respondents in our survey was the risk of venous thrombotic events, which is consistent with the concerns often discussed by authors of existing studies on TXA use. The safety of TXA has been studied in numerous patient populations and across various surgical specialties. TXA used for the treatment of postpartum hemorrhage has not been shown to increase the risk of thromboembolic events in the obstetric population (27). In studies of TXA use in gynecologic surgery, thromboembolic events have not been specifically examined as the primary outcome of interest. The safety of TXA has been illustrated in non-gynecologic fields such as orthopedic surgery and trauma patients; studies have shown no significant increased risk of thromboembolic events with use of TXA (28,29). Though numerous studies have highlighted the safety of TXA use in these various patient populations, prophylactic administration of TXA should only take place after careful assessment of underlying risk factors for thromboembolism in patients undergoing gynecologic surgery.
Survey respondents identified lack of surgeon knowledge/familiarity with TXA as the greatest barrier to use. Potential solutions to overcoming this barrier include: promoting surgical knowledge and familiarity through continual review of evidence-based practices and focused surgical education to enhance surgeon comfort and willingness to incorporate TXA into surgical practice. There is a mounting body of literature exploring the benefit of TXA in gynecologic surgery, however, the use of TXA has not yet become the standard of care in gynecologic surgery and there are no current perioperative guidelines specifically addressing indications for TXA administration. Another possible solution to increase TXA use is the development and implementation of perioperative protocols for prophylactic TXA use for specific gynecologic procedures and patients that are at greater risk for intraoperative hemorrhage.
Though TXA use in gynecology has been widely described in the literature, our study is the first to provide in-depth characterization of practice patterns and utilization of TXA among gynecologists. We also explored and identified potential barriers and limitations to use of TXA, which may shed additional insight into surgeon decision making and perioperative management. Additional strengths of our study include global representation from the AAGL membership, which includes the US and more than 100 countries outside of the US, and inclusion of respondents from diverse practice settings. The previously discussed survey of Canadian obstetrician-gynecologists did include a brief assessment of TXA use, however, only in the context of myomectomy surgery, and the total number of respondents (68 respondents) was lower than our survey which included 314 respondents (24).
A limitation of our study was the low response rate of 5.4%. Though the response rate was low given the large membership, the sample was representative of the membership population as a whole with similar rates of gender, location of practice and experience. Additionally, the total number of respondents in our study (314 respondents) is similar to a prior survey of AAGL membership that yielded 330 respondents but lower than a morcellation survey study by Lum et al. which included 615 respondents (30,31). To capture more respondents, our study included a follow-up reminder with a survey link published in the electronic AALG member newsletter. Other methods of improving response rates, which were not implemented in our study, include offering survey incentives or additional repeated reminders. Our survey design was entirely multiple-choice based and did not include open-ended questions. Assessment of provider barriers and concerns may have been more comprehensive and robust by not restricting answers to the multiple-choice selections and offering opportunities for respondents to write in responses to better elicit themes. Another limitation is the potential for response bias and our study results not accurately reflecting the precise utilization of TXA in clinical scenarios. Additionally, the practice patterns of members of AAGL, which is regarded as an organization focused on minimally invasive gynecologic surgery, may not be representative of obstetrician-gynecologists as a whole. We believe our study helps build upon the literature currently published and hope that future studies will improve upon this work and extend the generalizability of our findings.
Conclusions
In summary, our study investigated practice patterns and utilization of TXA among gynecologists and demonstrated that oral TXA prescribing was very common and its use was consistent among providers whereas considerable variability exists in the use of TXA for acute surgical bleeding and perioperative prophylaxis during myomectomy and hysterectomy. The results reported in this paper may be used to partially inform future prospective studies on the use of TXA in populations receiving gynecologic surgical care. As more studies emerge regarding the potential benefit and safety of TXA during gynecologic surgery, additional evidence may prompt greater acceptance among surgeons and aid in the identification of clinical scenarios in which TXA use is warranted.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SURGE reporting checklist. Available at https://gpm.amegroups.com/article/view/10.21037/gpm-22-12/rc
Data Sharing Statement: Available at https://gpm.amegroups.com/article/view/10.21037/gpm-22-12/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.com/article/view/10.21037/gpm-22-12/coif). LCY serves as an unpaid editorial board member of Gynecology and Pelvic Medicine. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The survey received exempt status from the Loyola University Chicago Health Sciences Division Institutional Review Board (IRB #211981) and was approved by the AAGL Research Committee and AAGL Board of Directors. Informed consent was not required since the research involved no more than minimal risk to subjects.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McPherson K, Metcalfe MA, Herbert A, et al. Severe complications of hysterectomy: the VALUE study. BJOG 2004;111:688-94. [Crossref] [PubMed]
- Clarke-Pearson DL, Geller EJ. Complications of hysterectomy. Obstet Gynecol 2013;121:654-73. [Crossref] [PubMed]
- Panteli M, Pountos I, Giannoudis PV. Pharmacological adjuncts to stop bleeding: options and effectiveness. Eur J Trauma Emerg Surg 2016;42:303-10. [Crossref] [PubMed]
- Callender ST, Warner GT, Cope E. Treatment of menorrhagia with tranexamic acid. A double-blind trial. Br Med J 1970;4:214-6. [Crossref] [PubMed]
- Shakur H, Elbourne D, Gülmezoglu M, et al. The WOMAN Trial (World Maternal Antifibrinolytic Trial): tranexamic acid for the treatment of postpartum haemorrhage: an international randomised, double blind placebo controlled trial. Trials 2010;11:40. [Crossref] [PubMed]
- Roberts I, Shakur H, Coats T, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess 2013;17:1-79. [Crossref] [PubMed]
- Kagoma YK, Crowther MA, Douketis J, et al. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res 2009;123:687-96. [Crossref] [PubMed]
- Henry DA, Carless PA, Moxey AJ, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev 2011;CD001886. [PubMed]
- Topsoee MF, Settnes A, Ottesen B, et al. A systematic review and meta-analysis of the effect of prophylactic tranexamic acid treatment in major benign uterine surgery. Int J Gynaecol Obstet 2017;136:120-7. [Crossref] [PubMed]
- Shaaban MM, Ahmed MR, Farhan RE, et al. Efficacy of Tranexamic Acid on Myomectomy-Associated Blood Loss in Patients With Multiple Myomas: A Randomized Controlled Clinical Trial. Reprod Sci 2016;23:908-12. [Crossref] [PubMed]
- Topsoee MF, Bergholt T, Ravn P, et al. Anti-hemorrhagic effect of prophylactic tranexamic acid in benign hysterectomy-a double-blinded randomized placebo-controlled trial. Am J Obstet Gynecol 2016;215:72.e1-8. [Crossref] [PubMed]
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol 2010;116:865-75. [Crossref] [PubMed]
- Ker K, Prieto-Merino D, Roberts I. Systematic review, meta-analysis and meta-regression of the effect of tranexamic acid on surgical blood loss. Br J Surg 2013;100:1271-9. [Crossref] [PubMed]
- Brenner A, Ker K, Shakur-Still H, et al. Tranexamic acid for post-partum haemorrhage: What, who and when. Best Pract Res Clin Obstet Gynaecol 2019;61:66-74. [Crossref] [PubMed]
- Matteson KA, Rahn DD, Wheeler TL 2nd, et al. Nonsurgical management of heavy menstrual bleeding: a systematic review. Obstet Gynecol 2013;121:632-43. [Crossref] [PubMed]
- ACOG committee opinion no. 557: Management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol 2013;121:891-6. [Crossref] [PubMed]
- James AH, Kouides PA, Abdul-Kadir R, et al. Evaluation and management of acute menorrhagia in women with and without underlying bleeding disorders: consensus from an international expert panel. Eur J Obstet Gynecol Reprod Biol 2011;158:124-34. [Crossref] [PubMed]
- Sallam HF, Shady NW. Reducing Blood Loss During Abdominal Hysterectomy with Intravenous Versus Topical Tranexamic Acid: A Double-Blind Randomized Controlled Trial. J Obstet Gynaecol India 2019;69:173-9. [Crossref] [PubMed]
- Opoku-Anane J, Vargas MV, Marfori CQ, et al. Intraoperative tranexamic acid to decrease blood loss during myomectomy: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol 2020;223:413.e1-7. [Crossref] [PubMed]
- Caglar GS, Tasci Y, Kayikcioglu F, et al. Intravenous tranexamic acid use in myomectomy: a prospective randomized double-blind placebo controlled study. Eur J Obstet Gynecol Reprod Biol 2008;137:227-31. [Crossref] [PubMed]
- Ngichabe S, Obura T, Stones W. Intravenous tranexamic acid as an adjunct haemostat to ornipressin during open myomectomy. A randomized double blind placebo controlled trial. Ann Surg Innov Res 2015;9:10. [Crossref] [PubMed]
- Pabinger I, Fries D, Schöchl H, et al. Tranexamic acid for treatment and prophylaxis of bleeding and hyperfibrinolysis. Wien Klin Wochenschr 2017;129:303-16. [Crossref] [PubMed]
- Sentilhes L, Sénat MV, Le Lous M, et al. Tranexamic Acid for the Prevention of Blood Loss after Cesarean Delivery. N Engl J Med 2021;384:1623-34. [Crossref] [PubMed]
- Nensi A, Yeung GWY, Frecker H, et al. Measures to Reduce Perioperative and Intraoperative Blood Loss at Myomectomy: A Survey of Obstetrician-Gynaecologists. J Obstet Gynaecol Can 2020;42:550-5. [Crossref] [PubMed]
- Guerriero C, Cairns J, Perel P, et al. Cost-effectiveness analysis of administering tranexamic acid to bleeding trauma patients using evidence from the CRASH-2 trial. PLoS One 2011;6:e18987. [Crossref] [PubMed]
- Li B, Miners A, Shakur H, et al. Tranexamic acid for treatment of women with post-partum haemorrhage in Nigeria and Pakistan: a cost-effectiveness analysis of data from the WOMAN trial. Lancet Glob Health 2018;6:e222-8. [Crossref] [PubMed]
- Shakur H, Beaumont D, Pavord S, et al. Antifibrinolytic drugs for treating primary postpartum haemorrhage. Cochrane Database Syst Rev 2018;2:CD012964. [Crossref] [PubMed]
- Lin ZX, Woolf SK. Safety, Efficacy, and Cost-effectiveness of Tranexamic Acid in Orthopedic Surgery. Orthopedics 2016;39:119-30. [Crossref] [PubMed]
- Guyette FX, Brown JB, Zenati MS, et al. Tranexamic Acid During Prehospital Transport in Patients at Risk for Hemorrhage After Injury: A Double-blind, Placebo-Controlled, Randomized Clinical Trial. JAMA Surg 2020;156:11-20. Erratum in: JAMA Surg 2021;156:105. [Crossref] [PubMed]
- Mikhail E, Scott L, Miladinovic B, et al. Association between Fellowship Training, Surgical Volume, and Laparoscopic Suturing Techniques among Members of the American Association of Gynecologic Laparoscopists. Minim Invasive Surg 2016;2016:5459147. [Crossref] [PubMed]
- Lum DA, Sokol ER, Berek JS, et al. Impact of the 2014 Food and Drug Administration Warnings Against Power Morcellation. J Minim Invasive Gynecol 2016;23:548-56. [Crossref] [PubMed]
Cite this article as: Bogue R, Wozniak A, Yang LC. A survey of gynecologists’ utilization of tranexamic acid and factors influencing prophylactic use of tranexamic acid. Gynecol Pelvic Med 2023;6:2.



