
The histological value of chemotherapy response score in advanced ovarian cancer—histopathological challenges
Introduction
The chemotherapy response score (CRS) (1) is used to assess response to neoadjuvant chemotherapy in the treatment of high-grade tubo-ovarian serous carcinomas and is useful to determine further management which may include interval debulking surgery (2). In particular, where the CRS is deemed to be CRS 3 (see below), it is recognised that there is a significant association with improved progression free survival (PFS) and overall survival (OS) in multivariate models adjusted for age and stage (3,4). One such meta-analysis shows a hazard ratio of 1.9 (for CRS 1/2) versus 1.0 (for CRS 3) in progression-free survival and a hazard ratio of 1.73 (for CRS1/2) versus 1.0 (for CRS 3) in overall survival (3). The same analytical paper indicates that patients with known germline BRCA1/2 status were more likely to achieve a CRS 3. Several studies have demonstrated the validity of the CRS system and the prognostic implications associated with it (4-7). Equally, studies have confirmed that the CRS system is highly reproducible (4,7,8) despite its purely morphological basis. As a result, there is significant interobserver agreement seen with CRS 3 in several of these studies. Such positivity of a system that is reproducible and is also a reliable prognostic indicator means that the onus is on the reporting pathologist to accurately assess tissue for effects of chemotherapy in high grade serous tubo-ovarian cancers. A more recent study has shown that the CRS, when used on the omentum and adnexa and as a combined score, was significantly associated with PFS but not with OS (9). In the same study, adnexal CRS1/2 were shown to be more likely to develop platinum-resistant disease. Many recent studies have used the CRS system to look at impact on survival in certain cohorts of patients (10,11).
The pivotal role of the pathologist is very clearly outlined in a review article by Williams and Ganesan (12). In addition, it has been shown that the CRS correlates very well with radiological assessment using CT imaging (13). The International Collaboration on Cancer Reporting (ICCR) has recommended incorporation of the CRS system into tubo-ovarian cancer reporting (14), and it has also been adopted into the dataset for reporting such cancers by the Royal College of Pathologists (15).
Histological features
Below are the histological features for each score in the 3-tier system:
- CRS 1 (no or minimal tumour response): mainly viable tumour with minimal regression-associated fibro-inflammatory changes limited to a few foci (Figure 1).
- CRS 2 (partial tumour response): multifocal or diffuse regression-associated fibro-inflammatory changes, with viable tumour ranging from diffuse sheets, streaks or nodules to extensive regression with multifocal but easily identifiable residual tumour (Figure 2).
- CRS 3 (near-complete or complete tumour response): mainly regression, with few irregularly scattered individual tumour cells or cell groups (all measuring <2 mm), or no residual tumour identified (Figure 3).
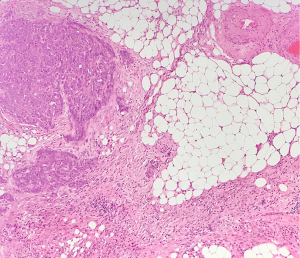
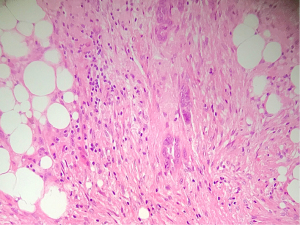
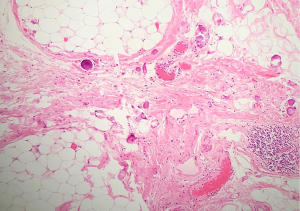
Fibro-inflammatory changes are denoted by fibrosis associated with macrophages including foam cells, mixed inflammation cells and Psammoma bodies. These are to be distinguished from tumour-related inflammation or desmoplasia.
The ICCR recommends that the scoring be carried out on a single haematoxylin and eosin-stained section of omentum and the section being assessed should be the one where ‘the least’ response to chemotherapy is seen. Generally, ancillary techniques, including special stains and immunohistochemistry, are not needed to help with the assessment of omentum.
There will be cases where no tumour is seen or any changes of response to chemotherapy identified. Before assigning a CRS of 3, it is considered necessary to check whether tumour had been present in the omentum prior to the start of the chemotherapy. For this, looking at radiological images would be important.
Histological challenges
- There is a possibility of interobserver variation, particularly for CRS 1 and CRS 2. However, one study, in particular, has shown that the interobserver agreement can improve after online training using the tool provided by the Genetic Pathology Evaluation centre (16). Apart from improving interobserver agreement by using online tools, it would be possible to discuss difficult cases as consensus meetings in order to assign CRS in cases where features are difficult to interpret. Further detailed work needs to look at combining CRS 1 and CRS 2 into one score and comparing PFS and OS rates against CRS 3.
- It would be necessary to ensure that the omentum is reasonably well sampled for histological examination, particularly in cases where the chemotherapy response appears to be significant. It is standard practice to take at least 10 sections as recommended by the ICCR (12). Assessment of omental blocks with little or no residual carcinoma may also require a consensus approach.
- In many cases one finds evidence of very good response in the omentum, rendering a CRS of 3 but, at the same time in the same patient, there may be bulky viable cancer in the pelvis. For a pathologist this becomes a difficult situation to reconcile where the features indicate CRS 3 in the omentum and CRS 1 in the pelvis. The study by Ditzel et al. (8) showed that the interobserver reproducibility was poor when assessed in adnexae and this did not improve after online training. This study also found that the combination of CRS 3 in both the ovary and the omentum resulted in a significantly longer PFS. The strong association shown between a CRS 3 in omental tissue and favourable survival rates means that assessment only in omental tissue is acceptable as a good prognostic tool. Further work could look into using a combined CRS system, rendering two scores—omental and adnexal—as part of the normal dataset of reporting. There may be instances when no tumour is seen in the omentum and pre-chemotherapy imaging confirms absence of any tumour in the omentum. In such situations, it may be necessary to offer a CRS on other tissues where a response is seen, applying the same principle of assessing tissue with ‘the least’ response.
- Very occasionally, only small core biopsies may be offered as part of assessing the chemotherapy response prior to consideration of debulking surgery. It is not ideal to assess such tissues because the biopsy may not be representative of what is happening in the rest of the omentum. One way around this is to take several core biopsies from different areas of the omentum. Most often, in such situations, imaging is likely to offer the better information on the state of response to chemotherapy.
Future direction
- More data needs to be collected with regard to a 3-tier versus a 2-tier CRS system.
- Further work needs to look at the value of combining scores of omental and adnexal/pelvic assessments using a 3-tier as well as a 2-tier system.
- Whichever CRS system is finally found to be not only easily reproducible but also a very reliable prognostic tool can possibly be used to assess the efficacy of other chemotherapeutic agents and survival rates.
Conclusions
The CRS system has been around for almost 11 years and more recently it has been incorporated as an important dataset item in cases of high-grade serous tubo-ovarian/peritoneal cancers. Training in assessing CRS helps to improve reproducibility and interobserver agreement and this can be strengthened by consensus reporting in difficult cases. There is a definite association between PFS/OS and CRS 3 in the omentum and, hence, assessment of omental tissue remains the current practice. More data is required to compare a 3-tier versus a 2-tier CRS system now that it is recognised that CRS1 with CRS 2 show less favourable survival rates, and at the same time, further data showing the impact of a combined CRS in omentum and adnexae would be useful for clinical practice.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Hooman Soleymani Majd) for the series “Evolutions in the Management of Advanced Ovarian Cancer” published in Gynecology and Pelvic Medicine. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-31/coif). The series “Evolutions in the Management of Advanced Ovarian Cancer” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Böhm S, Faruqi A, Said I, et al. Chemotherapy Response Score: Development and Validation of a System to Quantify Histopathologic Response to Neoadjuvant Chemotherapy in Tubo-Ovarian High-Grade Serous Carcinoma. J Clin Oncol 2015;33:2457-63. [Crossref] [PubMed]
- Böhm S, Le N, Lockley M, et al. Histopathologic response to neoadjuvant chemotherapy as a prognostic biomarker in tubo-ovarian high-grade serous carcinoma: updated Chemotherapy Response Score (CRS) results. Int J Gynecol Cancer 2019;29:353-6. [Crossref] [PubMed]
- Cohen PA, Powell A, Böhm S, et al. Pathological chemotherapy response score is prognostic in tubo-ovarian high-grade serous carcinoma: A systematic review and meta-analysis of individual patient data. Gynecol Oncol 2019;154:441-8. Erratum in: Gynecol Oncol 2020;157:558-9 Erratum in: Gynecol Oncol 2021;161:328-9. [Crossref] [PubMed]
- Lee JY, Chung YS, Na K, et al. External validation of chemotherapy response score system for histopathological assessment of tumor regression after neoadjuvant chemotherapy in tubo-ovarian high-grade serous carcinoma. J Gynecol Oncol 2017;28:e73. [Crossref] [PubMed]
- Singh P, Kaushal V, Rai B, et al. The chemotherapy response score is a useful histological predictor of prognosis in high-grade serous carcinoma. Histopathology 2018;72:619-25. [Crossref] [PubMed]
- Rajkumar S, Polson A, Nath R, et al. Prognostic implications of histological tumor regression (Böhm's score) in patients receiving neoadjuvant chemotherapy for high grade serous tubal & ovarian carcinoma. Gynecol Oncol 2018;151:264-8. [Crossref] [PubMed]
- Said I, Böhm S, Beasley J, et al. The Chemotherapy Response Score (CRS): Interobserver Reproducibility in a Simple and Prognostically Relevant System for Reporting the Histologic Response to Neoadjuvant Chemotherapy in Tuboovarian High-grade Serous Carcinoma. Int J Gynecol Pathol 2017;36:172-9. [Crossref] [PubMed]
- Ditzel HM, Strickland KC, Meserve EE, et al. Assessment of a Chemotherapy Response Score (CRS) System for Tubo-Ovarian High-Grade Serous Carcinoma (HGSC). Int J Gynecol Pathol 2019;38:230-40. [Crossref] [PubMed]
- Lawson BC, Euscher ED, Bassett RL, et al. A 3-Tier Chemotherapy Response Score for Ovarian/Fallopian Tube/Peritoneal High-grade Serous Carcinoma: Is it Clinically Relevant? Am J Surg Pathol 2020;44:206-13. [Crossref] [PubMed]
- Barrington DA, Felix AS, Owda R, et al. Pathologic chemotherapy response score in epithelial ovarian cancer: Surgical, genetic, and survival considerations. Surg Oncol 2020;34:40-5. [Crossref] [PubMed]
- Lee YJ, Kim HS, Rim JH, et al. Germline BRCA, chemotherapy response scores, and survival in the neoadjuvant treatment of ovarian cancer. BMC Cancer 2020;20:185. [Crossref] [PubMed]
- Williams AT, Ganesan R. Role of the pathologist in assessing response to treatment of ovarian and endometrial cancers. Histopathology 2020;76:93-101. [Crossref] [PubMed]
- McNulty M, Das A, Cohen PA, et al. Measuring response to neoadjuvant chemotherapy in high-grade serous tubo-ovarian carcinoma: an analysis of the correlation between CT imaging and chemotherapy response score. Int J Gynecol Cancer 2019; [Crossref] [PubMed]
- McCluggage WG, Judge MJ, Clarke BA, et al. Data set for reporting of ovary, fallopian tube and primary peritoneal carcinoma: recommendations from the International Collaboration on Cancer Reporting (ICCR). Mod Pathol 2015;28:1101-22. [Crossref] [PubMed]
- Wilkinson N, Vroobel K, McLuggage WG. Dataset for histopathological reporting of carcinomas and borderline tumours of ovaries, fallopian tubes and peritoneum 2019. Available online: https://www.rcpath.org
- Genetic Pathology valuation Centre. Chemotherapy response Score (CRS): Training Web site for CRS system. Available online: http://www.gpecimage.ubc.ca/aperio/images/crs
Cite this article as: Manek S. The histological value of chemotherapy response score in advanced ovarian cancer—histopathological challenges. Gynecol Pelvic Med 2023;6:6.


