
Vulvar neuroendocrine carcinoma: a case report and literature review
Introduction
Neuroendocrine neoplasms are rare diseases of the female genital tract. The most common type of neuroendocrine neoplasm of the female genital tract arises from the ovary and is a clinically benign carcinoid tumor. Neuroendocrine neoplasms of the vulva are extremely rare and have been reported only in case reports. Therefore, it presents a diagnostic challenge, and there is no consensus regarding their optimal treatment because of the rarity of reported cases. The World Health Organization (WHO) 2014 classification divides neuroendocrine neoplasms of the genital tract according to the tumor site and grade [low-grade neuroendocrine tumor (NET) and high-grade neuroendocrine carcinoma (NEC)] (1,2). High-grade neuroendocrine tumors of the vulva include small cell NEC, large cell NEC, and Merkel cell carcinoma (MCC). The revised 2020 WHO classification divides neuroendocrine neoplasms into NET, small cell neuroendocrine carcinoma (SCNEC), large cell neuroendocrine carcinoma (LCNEC), and carcinoma admixed with NEC (3). Our report describes a rare case of vulvar NEC and reviews the available literature on neuroendocrine neoplasms of the vulva to inform the clinical management of this rare tumor. Compared with similar cases that also with vulvar neuroendocrine carcinoma, our patient received only vulvar wide local excision with no postoperative therapy and had good outcome until the next 17 months follow-up time. We present the following case in accordance with the CARE reporting checklist (available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-62/rc).
Case presentation
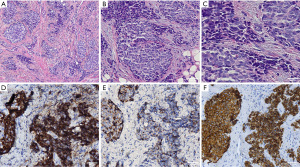
A 47-year-old woman presented to a local hospital with a 2-month history of a progressively enlarging vulvar mass in the left labium majus without purulent discharge. The patient’s previous medical history was normal. She first noticed the mass approximately 1 month prior in October, 2020 and sought treatment at a local hospital, where she underwent vulvar mass excision. The tumor was 1cm in diameter. Microscopically, the tumor was composed of sheets, nests, and cords of closely packed, oval to spindle, small cells with scanty cytoplasm (Figure 1A). The tumor cells have hyperchromatic nuclei with finely stippled chromatin, indistinct nucleoli, and a high nuclear to cytoplasmic ratio (Figure 1B,1C). Immunohistologically, tumor cells were positive for synaptophysin (Figure 1D), chromogranin (Figure 1E), and P-CK (Figure 1F), negative for CD56, HMB45, Melan-A, LCA. The pathological report was high-grade neuroendocrine carcinoma.
The patient then sought treatment at our hospital. Gynecological examination revealed swelling of the left labium majus and a scar from the previous excision surgery. A positron emission tomography and computed tomography (PET-CT) scan did not show any metastasis. Preoperative laboratory examinations were normal. We performed vulvar wide local excision on November 20th, 2020. The second pathological examination showed no residual tumor. The patient did not receive any postoperative therapy and was alive with no recurrence 17 months after the surgery. Pelvic and abdominal CT and lung CT showed no abnormalities in postoperative review till now. Close follow-up is still continued. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) (Number 2022026) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Neuroendocrine neoplasms arise in all sites of the female genital tract, including the cervix, endometrium, ovary, vagina, and vulva. Neuroendocrine neoplasms of the female genital tract are rare and comprise less than 2% of all genital malignancies (4). We searched the PubMed database for articles using the following search terms:“vulva” and “vulvar” combined with “neuroendocrine carcinoma”, “MCC”, “small cell carcinoma”, and “neuroendocrine neoplasia”. The included articles were limited to those published in the English language from the database’s inception to February 2021. Twenty-nine cases were identified using the PubMed search.
Due to their rarity, neuroendocrine neoplasms of the genital tract present a diagnostic challenge for both gynecologists and pathologists, and the evidence is insufficient to implement universal treatment guidelines. Table 1 presents the clinical characteristics of the 29 cases identified in the literature. There were 16 cases of MCC (5-20), five cases of neuroendocrine differentiation with other non-neuroendocrinecarcinoma (21-25), five cases of SCNEC (26-30), and three cases of non-specified type of neuroendocrine carcinoma (31-33). The time between the onset of symptoms and pathologic diagnosis ranged from 1 to 54 months. The age range at presentation was 28–92 years, and the average age was 57.2 years (Table 2). The mean tumor size was 4.2 cm (range, 1–9 cm). The labium majus, labium minus, Bartholin’s gland, paraclitoral site, and the vulva (not specified) were involved in 15, 3, 5, 2, and 4 of 29 cases, respectively in Table 2. The clinical presentation of the neuroendocrine carcinoma was a local mass (75.9%), bleeding (6.9%), and pruritus (10.3%). Eight cases presented with inguinal metastasis. Because of the nonspecific symptoms, pathological findings are important for diagnosing neuroendocrine carcinoma of the vulva. The WHO 2020 classification describes the histological and chemical characteristics of the neuroendocrine neoplasms of the genital tract; histologically, NETs are characterized by a variable amount of cytoplasm and small nucleoli with uniform chromatin (3). SCNEC comprises highly atypical cells, scant to indiscernible cytoplasm, and ovoid to slightly spindled nuclei with hyperchromatic and dispersed chromatin (3). LCNEC is characterized by moderate amounts of cytoplasm and large nuclei with coarse chromatin (3). Dense cored secretory granules surrounded by limiting membranes are visible on electron microscopy (34). Immunohistochemical markers can also help differentiate neuroendocrine tumors. Synaptophysin, chromatin, and CD56 are the most commonly identified neuroendocrine markers (3). CK20 is typically positive for MCC, and CK7 and TTF are typically negative, ruling out neuroendocrine metastatic carcinoma (35). In addition to that, immunohistochemistry for neuron-specific enolase or argyrophil stains can help to differentiate neuroendocrine carcinoma from other small cell carcinomas of the vulva such as lymphoma, melanoma, poorly differentiated carcinoma of the Bartholin gland, and neuroblastoma (7). It is also important that patients undergo CT or PET/CT scans to rule out metastases from other sites such as the lungs or other genital female tract. According to the National Comprehensive Cancer Network (NCCN) guidelines, 26–36% of cases of MCC present with lymph node involvement and 6–16% present with distant metastasis (36). Our review found that the incidence of lymph node metastases at presentation was 28.6%. Therefore, PET-CT should be considered in these patients because of the high rate of metastatic spread.
Table 1
| Author, year | Age (year) | Size (cm) | Site | Clinical findings | Treatment | Histology | Follow up |
|---|---|---|---|---|---|---|---|
| Tang et al. 1982 (5) | 67 | 1.5 | The left labium minus | Intermittent burning of the vulva | Wide local excision + radiotherapy |
Merkel cell carcinoma | 2 years: left groin LN metastasis(mets); 27 months: neck and right proximal femur, liver mets; 30 months: died |
| Bottles et al. 1984 (6) | 73 | No | The left labia majora | Chronic ulceration | Drug administration + vulvectomy + left inguinal lymphadenectomy |
Merkel cell carcinoma | 9 months: local raised tumor + left inguinal LN mets 11 months:death due to acute MI + cardiopulmanary vessel mets |
| Copeland et al. 1985 (7) | 59 | 6×8 | The left labium majus | 18- month history of a painful lump | Left hemivulvectomy and left inguinal lymphadenectomy + radiotherapy + vulvar lesion excision (8 months later) | Merkel cell carcinoma | 8 months: pulmonary mets and 2.5 cm local mets; 12 months: died |
| Husseinzadeh et al. 1988 (8) | 47 | 4.2×3 | The right labium majus + vaginal introit | 3-month history of right labial, groin swelling, vaginal discharge and pain + bilateral LN mets | Vulvectomy + bilaterallymphadenectomy + radiotherapy + local excision and chemotherapy (3 months later) | Merkel cell carcinoma | 3 months: local and distant mets; 6 months: death |
| Chandeying 1989 (9) | 28 | 4 | The right labium majus | 1-month history of a painless lump + right inguinal LN met | Radical vulvectomy and bilateral groin nodes dissection + radiotherapy | Merkel cell carcinoma | No follow up |
| Loret de Mola et al. 1993 (10) | 28 | 3×2.5 | The left paraclitoral | 1-month history of local tumor | Local excision + wide local excisionand left inguinal lymphadenectomy (2 months later) + chemotherapy (8 months later) | Merkel cell carcinoma | 8 months: liver mets; 20 months: death |
| Chen 1994 (11) | 68 | 3×2.5 | The left paraclitoral | 1-month history of local tumor | local excision + chemotherapy (10 months later) | Merkel cell carcinoma | 9 months: bilateral inguinal LN and liver mets; 17 months: death |
| Scurry et al. 1996 (12) | 68 | 4×3 | The Posterior fourchette and left labium minus | 5-month history of a painless lump in the left side of the groin + bilateral inguinal met | Radical vulvectomy,bilateral inguinal node dissection, and left pelvic lymphadenectomy + chemotherapy and Radiotherapy | Merkel cell carcinoma | 2 months: para-aortic lymph node mets; 5 months: Alive with Para- aortic met |
| Gil-Moreno et al. (13) | 74 | 9 | The labium majus | 3 to 4 months history of a tumor in the labium majus | wide local excision | Merkel cell carcinoma | Free of disease at 13 months |
| Fawzi et al. 1997 (14) | 78 | 5.5×4 | The right side of the vulva | 1-month history of perineal itching and discomfort + pulmonary LN met | Radical vulvectomy with bilateral groin node dissection | Merkel cell carcinoma | 20 days postoperative: died (right groin site broke down and died of bleeding) |
| Hierro et al. 2000 (15) | 76 | 2.5 | The left labium minus | A vulvar tumor of few weeks’ evolution | local excision + local excision and left inguinal lymphadenectomy and radiotherapy(two months later) | Merkel cell carcinoma | 2 months: local and nodal mets; 10 months: death |
| Pawar et al. 2005 (16) | 35 | 6×4 | The left labium majus | 1 week history of Painful swelling of the vulvar and a purulent discharge + left inguinal LN met | The pus was drained + antibiotics + local excision | Merkel cell carcinoma | No follow up |
| Khoury-Collado et al. 2005 (17) | 49 | 2 | The right Bartholin’s gland | Pain and swelling on the right side of the vulva | Radical wide local excision and bilateral inguinal lymph node dissection + radiothearpy | Merkel cell carcinoma | Alive at 2 years |
| Mohit et al. 2009 (18) | 50 | 3–4 | The left labia majus | 3-month history of vulvar mass | local excision + radiotherapy and radical vulvectomy (2 months later) + chemotherapy (9 months later) |
Merkel cell carcinoma | 2 months: local recurrence mass; 11 months: death due to pulmonary embolism |
| Sheikh et al. 2010 (19) | 63 | 5×7 | The right labium majus | Bleeding and local mass | Wide local excision | Merkel cell carcinoma | 2 months: local + distantmets + death |
| Iavazzo et al. 2011 (20) | 63 | 9 | The left labium majus | A lump of the left labium of the vulva + left LN met | Radical vulvectomy + radiotherapy | Merkel cell carcinoma | No follow up |
| Rahilly et al. 1995 (21) | 69 | 4×3 | The right labium majus | 2-month history of vulvar itch and ulcerated swelling | Right hemivulvectomy | Mucinous adenocarcinoma with neuroendocrine differentiation | Alive at 2 years and 9 months with no recurrence |
| Graf et al. 1998 (22) | 75 | 4.5×4 | The left major labium | Genital bleeding | Radical vulvectomy with bilateral inguinal and femoral lymph lode dissection | Mucinous adenocarcinoma with neuroendocrine differentiation | Alive at four year follow up with no recurrence |
| Zhang et al. 2012 (23) | 40 | 3.5×2.7 | The left vulva | 3-month history of a painless left vulvar mass | Local excision | Solid papillary carcinoma with neuroendocrine differentiation | Alive at four year follow up with no recurrence |
| van Rosmalen et al. 2016 (24) | 92 | 8 | The left major labium | 10-year history of a tumor progressively increased in size the last months | Partial vulvectomy and resection of the PET positive inguinal lymph node | Mucinous adenocarcinoma with neuroendocrine differentiation | Alive at 15 months with no recurrence |
| Gabrilovich et al. 2017 (25) | 60 | 1.5 to 2 | The right labia majora | 2 to 3 months history of a small bump on her right vulva + LN met | Right radical wide local vulvar excision with right inguinofemoral lymph node dissection + radiotherapy + chemotherapy | Adenocarcinoma with neuroendocrine differentiation | Alive at 8 months with no recurrence |
| Jones et al. 1990 (26) | 30 | 2 | In the left Bartholin’s gland | 3-month history of progressively enlarging painful vaginal lump, dispareunia and left inguinal LN met | Local excision + diagnostic laparoscopy and inguinal node biopsy + chemotherapy | Small cell neuroendocrine carcinoma | No follow up |
| Cliby et al. 1991 (27) | 35 | Less than 1 | The right hemivulva | 2 months history of a vulvar mass | Right modified radical hemivulvectomy and bilateral superficial inguinal lymphadenectomy | Small cell neuroendocrine carcinoma | Alive at 21 months with no recurrence |
| Obermair et al. 2001 (28) | 49 | 3.5×2 | The right Bartholin’s gland | 5-month history of local swelling | Local excision | Small cell neuroendocrine carcinoma | Died 15 months, disseminated disease |
| Correia et al. 2017 (29) | 70 | No | Both labia | Gradual evolution pruritus gets worse | First:superficial vulvectomy; Then: radical vulvectomy and bilateral lymphadenectomy + right LN met )radiotherapy | High-grade small cell neuroendocrine carcinoma | No follow up |
| Jamshidi et al. 2020 (30) | 53 | 4×2.1 | The Bartholin’s gland | Several weeks of pain at the vaginal | First: bartholinectomy; Then: wide radical vulvectomy and vaginectomy and sentinel lymphadenectomy + radiotherapy + chemotherapy + irinotecan and sterotactic body radiation (1 year later) + a left frontal craniotomy + whole-brain radiation (1 year later) | Small cell neuroendocrine carcinoma | 1 years: liver mets; 3 years: brain mets |
| Nuciforo et al. 2004 (31) | 62 | 2 | The right labia majora | Soft, painful lump | Local excision + radical vulvectomy and radiotherapy (three months later) | Neuroendocrine carcinoma | Three months :local recurrence and LN mets; Alive at 19 months with multiple abdominal and thoracic metastases |
| Aminimoghaddam et al. 2016 (32) | 44 | 5×5 | The left labium major | A mass in the vulva | Left hemivulvectomy and bilateral lymph node dissection | Neuroendocrine carcinoma | Alive at Four year follow up with no recurrence |
| Wu et al. 2018 (33) | 56 | 3 | At the left Bartholin’s gland | 1- month history of increasing pain and swelling with bleeding | Radical wide local excision and bilateral inguinal lymph node dissection and chemotherapy + hepatic lobectomy and chemotherapy (1 month later) | Neuroendocrine carcinoma | Alive at 6 months with no local and distant metastasis |
LN, lymph node.
Table 2
| Characteristic | Number of the patients (%) or mean [range] |
|---|---|
| Mean age (years) | 57.2 [28–92] |
| Mean tumor diameter (cm) | 4.2 [1–9] |
| Location | |
| Labium minus | 3 (10.3) |
| Labia majora | 15 (51.7) |
| Bartholin’s gland | 5 (17.2) |
| Paraclitoral | 2 (6.9) |
| Vulva (not specified) | 4 (13.8) |
| Clinical presentation | |
| Local mass | 22 (75.9) |
| Lymph node metastasis | 8 (27.9) |
| Bleeding | 2 (6.9) |
| pruritus | 3 (10.3) |
| Ulceration | 2 (6.9) |
| Intermittent burning | 1 (3.4) |
| Surgical options | |
| Local excision | 5 (17.2) |
| Wide local excision | 7 (24.1) |
| Hemivulvectomy | 3 (10.3) |
| Partial vulvectomy | 1 (3.4) |
| Vulvectomy | 2 (6.9) |
| Radical vulvectomy | 11 (37.9) |
| Inguinofemoral lymph node dissection | |
| Yes | 17 (58.6) |
| No | 12 (41.4) |
| Ajuvant therapy | |
| Yes | 17 (58.6) |
| Radiotherapy | 8 (47.1) |
| Radiotherapy + chemotherapy | 5 (29.4) |
| Chemotherapy | 4 (23.5) |
| No | 12 (41.4) |
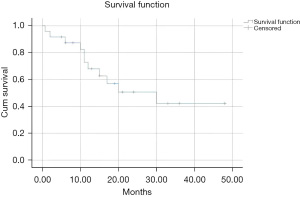
Table 2 presents the treatment data for the included articles. The type of surgery, adjuvant therapy, outcome, and survival outcomes are presented in detail. Inguinal lymph node dissection was reported in 17 cases. Adjuvant therapy (including radiotherapy, chemotherapy, radiotherapy, and chemotherapy) was reported in 17 patients. The first line treatment for neuroendocrine carcinoma is surgical excision (35). The 2021 guidelines of the European Society of Gynaecological Oncology (ESGO) for vulvar cancers recommend radical local excision with <1 cm surgical free excision margins. For tumors >pT1a, groin treatment should be performed. Patients with unifocal tumors<4 cm in size with no suspicious groin nodes are suitable for the sentinel lymph node procedure (37). For patients with tumors of ≥4 cm and/or in cases of multifocal invasive disease, inguinofemoral lymphadenectomy is recommended (37). The 2018 NCCN Guidelines for MCC recommend wide local excision with 1–2 cm margins with sentinel lymph node biopsy (36). Unfortunately, there are no clear guidelines for treating neuroendocrine neoplasms of the vulva. The adjuvant radiation and/or chemotherapy is a reasonable consideration because of the aggressive performance of this disease in the vulva. Figure 2 shows the Kaplan-Meier survival curve of 29 patients with vulvar neuroendocrine carcinoma included in the literature. The median survival time of included patients was 30 months. The 3-year survival rate was 42.2%. A total of 13/29 (46.4%) patients developed recurrence from 2–15 months; this was consistent with the recurrence of MCC, which has been reported to range from 25–50% (38). The 2018 NCCN Guidelines for MCC recommend wide excision without adjuvant therapy for cases with negative resection margins and no high-risk features. Otherwise, adjuvant radiation should be performed (36). The 2021 ESGO guidelines for vulvar cancer recommend postoperative radiotherapy in cases of invasive disease extending to or close to the pathological excision margins of the primary tumor, with>1 metastatic lymph node, and/or with extracapsular lymph node involvement (37).
The etiology of the neuroendocrine carcinoma was reported to be associated with old age, immunosuppression, European ancestry, and ultraviolet radiation (39). Genetic abnormalities were found in MCC cell lines, and less genomic aberration is associated with better survival (19). Mohit et al. reported that the MCC of the vulvar was more aggressive than that in other places (18). Postoperative monitoring is necessary for patients with neuroendocrine neoplasia. Approximately 9–19% of patients diagnosed with MCC subsequently developed another malignancy, and half of the patients experienced recurrence in the first three years (40,41). Close surveillance was recommended immediately after treatment, and PET/CT is useful in monitoring recurrence.
Our study demonstrates that wide local excision with negative margins may be sufficient for the treatment of the high-grade vulvar neuroendocrine carcinoma. This is not consistent with the high aggressive behavior of the disease in the previous reports. However, the data in our study regarding the disease is still limited to support the conclusion. An overview of the existing literature also helps clinicians understand the disease better.
Conclusions
In summary, vulvar neuroendocrine carcinoma is a rare disease. Our case and the reviewed cases further our understanding of the clinical presentation, diagnosis, and treatment of this rare disease. However, current studies include a limited number of cases and short follow-up; therefore, future studies with more cases are warranted to help establish new treatment guidelines.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-62/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-62/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s)(Number 2022026) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kurman RJ, Carcangiu ML, Herrington CS, et al. WHO classification of tumours of the female reproductive organs. Lyon: IARC press; 2014.
- Howitt BE, Kelly P, McCluggage WG. Pathology of Neuroendocrine Tumours of the Female Genital Tract. Curr Oncol Rep 2017;19:59. [Crossref] [PubMed]
- Cree I A, White VA, Indave BI, et al. WHO classification of tumours of the female reproductive organs. Lyon: IARC press; 2020.
- Rouzbahman M, Clarke B. Neuroendocrine tumors of the gynecologic tract: select topics. Semin Diagn Pathol 2013;30:224-33. [Crossref] [PubMed]
- Tang CK, Toker C, Nedwich A, et al. Unusual cutaneous carcinoma with features of small cell (oat cell-like) and squamous cell carcinomas. A variant of malignant Merkel cell neoplasm. Am J Dermatopathol 1982;4:537-48. [Crossref] [PubMed]
- Bottles K, Lacey CG, Goldberg J, et al. Merkel cell carcinoma of the vulva. Obstet Gynecol 1984;63:61S-5S. [PubMed]
- Copeland LJ, Cleary K, Sneige N, et al. Neuroendocrine (Merkel cell) carcinoma of the vulva: a case report and review of the literature. Gynecol Oncol 1985;22:367-78. [Crossref] [PubMed]
- Husseinzadeh N, Wesseler T, Newman N, et al. Neuroendocrine (Merkel cell) carcinoma of the vulva. Gynecol Oncol 1988;29:105-12. [Crossref] [PubMed]
- Chandeying V, Sutthijumroon S, Tungphaisal S. Merkel cell carcinoma of the vulva: a case report. Asia Oceania J Obstet Gynaecol 1989;15:261-5. [Crossref] [PubMed]
- Loret de Mola JR, Hudock PA, Steinetz C, et al. Merkel cell carcinoma of the vulva. Gynecol Oncol 1993;51:272-6. [Crossref] [PubMed]
- Chen KT. Merkel's cell (neuroendocrine) carcinoma of the vulva. Cancer 1994;73:2186-91. [Crossref] [PubMed]
- Scurry J, Brand A, Planner R, et al. Vulvar Merkel cell tumor with glandular and squamous differentiation. Gynecol Oncol 1996;62:292-7. [Crossref] [PubMed]
- Gil-Moreno A, Garcia-Jiménez A, González-Bosquet J, et al. Merkel cell carcinoma of the vulva. Gynecol Oncol 1997;64:526-32. [Crossref] [PubMed]
- Fawzi HW, Cross PA, Buckley CH, et al. Neuroendocrine (Merkel cell) carcinoma of the vulva. J Obstet Gynaecol 1997;17:100-1. [Crossref] [PubMed]
- Hierro I, Blanes A, Matilla A, et al. Merkel cell (neuroendocrine) carcinoma of the vulva. A case report with immunohistochemical and ultrastructural findings and review of the literature. Pathol Res Pract 2000;196:503-9. [Crossref] [PubMed]
- Pawar R, Vijayalakshmy AR, Khan S, et al. Primary neuroendocrine carcinoma (Merkel's cell carcinoma) of the vulva mimicking as a Bartholin's gland abscess. Ann Saudi Med 2005;25:161-4. [Crossref] [PubMed]
- Khoury-Collado F, Elliott KS, Lee YC, et al. Merkel cell carcinoma of the Bartholin's gland. Gynecol Oncol 2005;97:928-31. [Crossref] [PubMed]
- Mohit M, Mosallai A, Monabbati A, et al. Merkel cell carcinoma of the vulva. Saudi Med J 2009;30:717-8. [PubMed]
- Sheikh ZA, Nair I, Vijaykumar DK, et al. Neuroendocrine tumor of vulva: a case report and review of literature. J Cancer Res Ther 2010;6:365-6. [Crossref] [PubMed]
- Iavazzo C, Terzi M, Arapantoni-Dadioti P, et al. Vulvar merkel carcinoma: a case report. Case Rep Med 2011;2011:546972. [Crossref] [PubMed]
- Rahilly MA, Beattie GJ, Lessells AM. Mucinous eccrine carcinoma of the vulva with neuroendocrine differentiation. Histopathology 1995;27:82-6. [Crossref] [PubMed]
- Graf AH, Su HC, Tubbs RR, et al. Primary neuroendocrine differentiated mucinous adenocarcinoma of the vulva: case report and review of the literature. Anticancer Res 1998;18:2041-5. [PubMed]
- Zhang C, Quddus MR, Sung CJ, et al. Vulvar encapsulated solid papillary carcinoma with neuroendocrine differentiation: a case report. Int J Surg Pathol 2012;20:97-100. [Crossref] [PubMed]
- van Rosmalen MH, Reijnen C, Boll D, et al. Vulvar mucinous adenocarcinoma with neuroendocrine differentiation: A case report and review of the literature. Pathol Res Pract 2016;212:234-7. [Crossref] [PubMed]
- Gabrilovich S, Cracchiolo B, Heller DS. Vulvar Adenocarcinoma With Neuroendocrine Differentiation: A Case Report. J Low Genit Tract Dis 2017;21:e23-5. [Crossref] [PubMed]
- Jones MA, Mann EW, Caldwell CL, et al. Small cell neuroendocrine carcinoma of Bartholin's gland. Am J Clin Pathol 1990;94:439-42. [Crossref] [PubMed]
- Cliby W, Soisson AP, Berchuck A, et al. Stage I small cell carcinoma of the vulva treated with vulvectomy, lymphadenectomy, and adjuvant chemotherapy. Cancer 1991;67:2415-7. [Crossref] [PubMed]
- Obermair A, Koller S, Crandon AJ, et al. Primary Bartholin gland carcinoma: a report of seven cases. Aust N Z J Obstet Gynaecol 2001;41:78-81. [Crossref] [PubMed]
- Correia A, Branco EC, Correia P, et al. Small Cell Carcinoma of the Vulva: Case Report. Clin Pract 2017;7:918. [Crossref] [PubMed]
- Jamshidi AM, Eichberg DG, Gultekin S, et al. Brain Metastasis From Bartholin Gland Carcinoma. World Neurosurg 2020;143:280-4. [Crossref] [PubMed]
- Nuciforo PG, Fraggetta F, Fasani R, et al. Neuroendocrine carcinoma of the vulva with paraganglioma-like features. Histopathology 2004;44:304-6. [Crossref] [PubMed]
- Aminimoghaddam S, Maghsoudnia A, Shafiee S. Moderately Differentiated Neuroendocrine Cell Carcinoma of the Vulva: A Case Report and Review of the Literature. Gulf J Oncolog 2016;1:72-5. [PubMed]
- Wu JC, Xi ML, Wang YQ, et al. Primary small cell neuroendocrine carcinoma of the Bartholin's gland: A case report. Oncol Lett 2018;16:4434-8. [Crossref] [PubMed]
- Osamura RY, Kumaki N, Kajiwara H, et al. Immunohistochemistry and Electron Microscopy for Diagnosis of Neuroendocrine Tumors. Pathology Case Reviews 2002;7:193-200. [Crossref]
- Nguyen AH, Tahseen AI, Vaudreuil AM, et al. Clinical features and treatment of vulvar Merkel cell carcinoma: a systematic review. Gynecol Oncol Res Pract 2017;4:2. [Crossref] [PubMed]
- Bichakjian CK, Olencki T, Aasi SZ, et al. Merkel Cell Carcinoma, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:742-74. [Crossref] [PubMed]
- European Society of Gynecological Oncology (ESGO) Guidelines. Vulvar Cancer Guidelines. Available online: https://guidelines.esgo.org/media/2016/08/ESGO-Vulvar-cancer-Complete-report-fxd2.pdf (accessed on 30 January 2021).
- Xue Y, Thakuria M. Merkel Cell Carcinoma Review. Hematol Oncol Clin North Am 2019;33:39-52. [Crossref] [PubMed]
- Rockville Merkel Cell Carcinoma Group. Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis, and clinical management. J Clin Oncol 2009;27:4021-6. [Crossref] [PubMed]
- Reichgelt BA, Visser O. Epidemiology and survival of Merkel cell carcinoma in the Netherlands. A population-based study of 808 cases in 1993-2007. Eur J Cancer 2011;47:579-85. [Crossref] [PubMed]
- Koljonen V, Kukko H, Tukiainen E, et al. Second cancers following the diagnosis of Merkel cell carcinoma: a nationwide cohort study. Cancer Epidemiol 2010;34:62-5. [Crossref] [PubMed]
Cite this article as: Yin Y, Han L, Chen Y, Ruan J, Zheng A. Vulvar neuroendocrine carcinoma: a case report and literature review. Gynecol Pelvic Med 2022;5:30.


