
Risk of minimal access surgery in uterine leiomyosarcomas: a narrative review
Introduction
Myometrial lesions and mini-invasive surgery
Benign myometrial lesions [leiomyoma (LM), myoma, fibromyoma or fibroid] are the first cause of gynaecological surgery between women aged 35 to 50 (1). They are often, but not always, symptomatic due their volume, giving abnormal uterine bleeding, dysmenorrhea, pelvic pain, vesical pressure and LMs are found in 10% of patients with an history of infertility (2).
After medical treatments failure, the first option is surgery and the treatments should be offer to the patient depending on her desire of pregnancy, site and volume of myomas. Myomectomy is a surgical approach to treat symptomatic uterine fibroids in women desiring fertility-sparing procedures while hysterectomy is performed in perimenopausal patients. Historically it was performed with a laparotomic access. Actually, both procedures are offered to the patient by mini-invasive approach.
Gynaecologists were pioneers in laparoscopy and advantages of mini-invasive surgery are well known. In almost all series reported in literature concerning myomectomy or hysterectomy, parameters as hospital stay, blood loss, wound infection or dehiscence, postoperative pain and recovery time are significantly reduced compared to laparotomic approach (3,4). Regarding hysterectomy, also the vaginal approach demonstrated good results, but it’s not indicated in enlarged uterus (5). According to some studies, exclusion criteria for laparoscopic myomectomy are the presence of more than four fibromas to remove in different uterine regions (in order to avoid multiple uterine incisions) and maximal mass diameter 12 cm on preoperative evaluation (6). In the event of large fibroids and/or large uterus treated with a mini-invasive approach, the tissue needs to be fragmentated in small parts to permit the removal through the small incisions. In case of hysterectomy, a vaginal morcellation with scalpel can be performed. In the setting of laparoscopic/robotic myomectomies (or supracervical hysterectomies) an intrabdominal morcellation is usually performed and the small pieces are taken off from the biggest trocars. In all cases the removal through some centimetres laparotomic access with manual morcellation is an alternative, but partially nullifying the benefits of mini-invasive techniques in term of possible complications and aesthetic results. Transvaginal morcellation through a posterior colpotomy has also been described. To avoid mini-laparotomic approach and hand mechanical morcellation with scalpel, in 1993 electronic morcellation (or power morcellation) system were introduced and began to be widely used. This equipment cuts the specimen in small pieces with electrical energy, reducing operative times. The procedure gained success worldwide and became the main system used in laparoscopic myomectomy/hysterectomy for benign conditions. After some incidental findings of occult malignancies morcellated, arose doubts in literature about its implications, as recurrence risk, upstaging and survival outcomes or possible obstacle to the pathologist interpretations and diagnosis. Regarding myometrial masses, on the other hand, despite the progresses in gynaecological ultrasound and magnetic resonance, the differential diagnosis between leiomyosarcoma (LMS) and myoma on preoperative evaluation, remains still challenging. Consequently, some patients with LMS could be treated initially with conservative surgery.
Uterine sarcomas
LMS is an aggressive malignant tumour and represents nearly 70% of all uterine sarcomas and 1.5% of all uterine cancers (7). Endometrial stromal sarcoma (ESS), adenosarcoma and undifferentiated endometrial sarcomas are different histological entities. Several histologic variants of LM and a premalignant form were described. The so called “smooth muscle tumours of uncertain malignant potential (STUMP)” is characterized by cytologic atypia but its risk of recurrence is debated and prognosis is unclear (8,9).
LMS frequently recurs and tends to locally spread or to send distant metastases, mostly to lungs. The median age at diagnosis of LMS is 54 years and risk factors are black race, previous pelvic irradiation, Li-Fraumeni and hereditary retinoblastoma genetic syndrome (10). As previously discussed in this number, these tumours tend to have a weak response to adjuvant therapies (11).
The prognosis is principally related to the stage of disease, with a 66% overall 5 years survival reported (12). Norwegian data shown even worse results, with a 5-year overall survival no better than 51% in stage I tumours and 25% in stage II (13).
Staging for LMS and ESS are described in Table 1.
Table 1
| Stage | Leiomyosarcomas and endometrial stromal sarcomas |
|---|---|
| I | Tumor limited to uterus |
| IA | Less than 5 cm |
| IB | More than 5 cm |
| II | Tumor extends beyond the uterus, within the pelvis |
| IIA | Adnexal involvement |
| IIB | Involvement of other pelvic tissues |
| III | Tumor invades abdominal tissues (not just protruding into the abdomen) |
| IIIA | One site1 |
| IIIB | More than one site |
| IIIC | Metastasis to pelvic and/or para-aortic lymph nodes |
| IVA | Tumor invades bladder and/or rectum |
| IVB | Distant metastasis |
FIGO, International Federation of Gynecology and Obstetrics.
In 2014, an FDA statement (than with revisions in 2017 and 2020) banned the use of power morcellation in peri-/post-menopausal patients and in “candidates for en bloc tissue removal” in order to avoid the risk of spreading neoplastic cell in the abdomen (14). These restrictions severely reduce the indications to mini-invasive myomectomy or hysterectomy for women with fibroids and they also instigated a debate on medical-legal aspects. As consequence an 11% increase of abdominal myomectomy was observed in the following years, with a parallel increment in surgical complications and hospital readmissions (15).
An Italian survey on the argument in 2016 showed that 58.7% of surgeons changed their approach in the fear of litigation (16).
There have also been more reports by gynaecologists on confined-in-bag morcellation, a technique in which specimens are located into a bag to permit a protect vaginal or abdominal fragmentation, avoiding peritoneal cells spread. This could be probably safer, but requires a little longer operative time and major costs. The different techniques are later described. We present the following article in accordance with the Narrative Review reporting checklist (available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-11/rc).
Methods
we reviewed principal evidences reported in literature in English by consulting the PubMed, Medline, and Cochrane Systematic Review database on occult sarcoma morcellation, using the key words occult uterine sarcoma, morcellation, mini-invasive surgery, LMS, morcellation injuries, mostly after 2014 FDA warning on the issue. Aims of the authors are to clarify incidence and oncological outcomes in the event of unsuspected LMS dissemination, description of surgical techniques for specimens’ removal and morcellation (unprotect or in bag), iatrogenic complications, preoperative clinical and instrumental features of patients not eligible for morcellation. We tried to simplify the debated aspects through the most cited articles from different countries in a narrative review.
Occult LMS incidence
The real occult sarcoma incidence in the setting of gynaecological surgery for benign condition varies widely in literature, often mixing premalignant masses or different histopathology.
Citing the 2014 FDA warning “approximately 1 in 350 women undergoing hysterectomy or myomectomy for the treatment of fibroids is found to have an unsuspected uterine sarcoma, a type of uterine cancer that includes leiomyosarcoma”. The reported LMS incidence is 1/498 (14). This data has been deeply criticized because referees to uterine sarcoma in general and come from retrospective small studies. Moreover, the cohort of women analysed was composed by different ages, unstratified for risk factors or candidates for various surgical treatments.
Seidman et al. observed that unexpected diagnoses of LM variants or atypical and malignant smooth muscle tumors had occurred in 1.2% of myometrial lesions clinically presumed to be “fibroids” (17). These percentages are debated. In a prospective multicentric study assessing different gynaecological and obstetrical outcomes of laparoscopic myomectomy, Sizzi and collaborators have found 2 cases of uterine sarcoma out of 2,050 procedures (0.09%) (18). A similar low incidence was reported by Mettler et al. They registered unsuspected uterine sarcomas in 1 of every 2,269 patients (0.044%) among those who underwent hysteroscopic, laparoscopic, but including also open myomectomies or hysterectomies to treat presumed benign uterine fibroids (19). Based on the 2017 Agency for Healthcare Research and Quality (AHRQ) report, patients may be informed that the risk of unexpected LMS may range from less than 1 in 770 surgeries to 1 in 10,000 surgeries for presumed symptomatic LMs (20). Understandably, the great variability of these data depends on the court of patients included (for example all the patients treated for fibroids with every kind of surgery including hysteroscopy) and often are mixed LMS, all uterine sarcomas, other malignancies or also premalignant diseases (in particular STUMP)/atypical myomas. Some incidence of occult sarcoma reported in literature are summarized in Table 2.
Table 2
| Year | Authors | % | Note |
|---|---|---|---|
| 2007 | Sizzi et al. | 0.09 | 2/2,050 uterine sarcomas in laparoscopic myomectomy (18) |
| 2012 | Seidman et al. | 1.2 | including premalignant disease (1 ESS and 1 LMS/1091 uterine morcellation) (17) |
| 2014/2017 | FDA | 0.28 | All uterine sarcomas 1/352—only leiomyosarcoma 1/498, reviewed in 2017: 1/570–750 (14) |
| 2018 | Pritts et al. | 0.11 | Meta-analysis, 32 LMS/30,193 patients, in prospective studies maybe lower (1/8,300) (21) |
| 2015 | Nugent et al. | 0.025–0.069 | LMS, 1/3,906 myomectomies—1/1,465 laparoscopic myomectomies or supracervical hysterectomies (35,161 patients) (22) |
| 2015 | Lieng et al. | 0.021 | 1 LMS/4,791 laparoscopic myomectomies or supracervical hysterectomies (23) |
| 2017 | Mettler et al. | 0.044 | 1 ESS/2,269 hysteroscopic, laparoscopic, laparotomic surgery for presumed fibroids (19) |
| 2017 | AHRQ | 0.02-0.08 | Risk of LMS in 160 meta-analysis, 136,195 patients (20) |
| 2020 | Gitas et al. | 0.24 | 4 (2 LMS + 2 ESS)/1,683 patients treated with electronical morcellation (24) |
| 2018 | Bretthauer et al. | 0.36 | 3,6 uterine sarcomas/1,000 laparoscopic hysterectomies -retrospectively from cancer national registers (25) |
LMS, leiomyosarcoma; ESS, endometrial stromal sarcoma.
Debates are also ongoing on the impact of sarcoma morcellation on oncological outcomes and common consensus are difficult to achieve. LMS is often an “incidental” diagnosis after fibroids treatments that could have caused tumour damage itself, due to surgical manipulation or cut-through. Nevertheless, prognosis is poor despite further treatments but after unprotect morcellation seems to be even worse. These facts are analysed in next paragraphs.
Surgery and morcellation techniques
Laparoscopic/robotic surgery for myomas are a minimally invasive techniques usually performed by expert Surgeons, although laparotomic surgery is still preferred in selected patients. The choice to remove myometrial masses or enlarged uterus by these approaches, depends on the surgeon experience, the size and location of presumed myomas, patients’ characteristics (age, obesity)/wishes and suspicion of malignancies (4,26).
Myomas are the first indication to perform a hysterectomy, but many women wish to preserve their fertility and choose conservative treatment, although the benefits of those on reproductive function are controversial (27). Preoperative assessment includes alternative medical treatments proposal if eligible and anaemia correction if present.
For laparoscopic myomectomy the patient is positioned in Trendelenburg, so optimal pulmonary ventilation is required. After pneumoperitoneum induction, usually four 5–12 mm trocars are collocated, myomas are detected and enucleated following their capsules. Then the myometrium is sutured (Figure 1). In case of big masses, the extraction can be obtained through morcellation, that can be usually achieved by abdominal or by vaginal accesses.
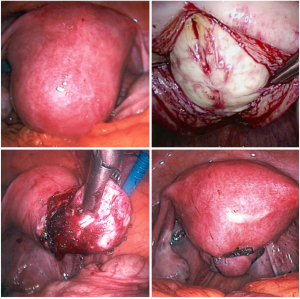
The specimens can be fragmentated with scissors or scalpels (hand morcellation) or with electric systems usually positioned in the umbilical trocar (power morcellation). Another option is to perform a suprapubic or periumbilical mini-laparotomy. Hand morcellation and mini-laparotomy are usually facilitated by wound retractors.
Vaginal morcellation can be easily performed during laparoscopic/robotic hysterectomy, where the uterus is reduced into smaller pieces with scissors or scalpels and extracted through the upper vaginal rim (28). This is possible also during laparoscopic myomectomy but is necessary to perform a culdotomy, usually through the posterior fornix (29-31). A cohort multicenter study published in 2018 involving 316 women, reported no pelvic infection, vaginal dehiscence, or complaints of dyspareunia at the 30-day follow-up (31).
As seen, morcellation gives the advantage to remove big specimens through small incisions, but with some implications. The principal criticisms to the technique are longer operative time and the tissue injuries, that could spread uterine cells in abdomen and also complicate the pathologist analysis.
Moreover, dissemination leads to possible peritoneal implants and parasitic myomas formation or malignancies diffusion.
Many authors proposed the use of contained bag system to reduce these complications. During laparoscopic/robotic surgery, a sterile bag is introduced through a trocar and a “pneumosac” is obtained. Contained bag system is equipped with two or three accesses that allow the introduction of the optical and the power morcellator. Fragmentation is performed directly into the bag and small specimens are removed. Finally, the pneumosac is released, and the bag is token away.
Another possibility is to place the specimens into a bag and to perform a hand in-bag morcellation through the abdomen or the vagina, with valves exposition.
According to some Authors anyway, disadvantages are worse abdominal organ visualization when bag is insufflated, longer operative time and major costs (even if this could be a bias depending on surgeons’ experience). Moreover, incidental bag rupture and indirect dissemination is possible and data on this event are missing (32).
Mechanical trauma morcellation of other organs are also reported in literature. These complications are descripted further ahead.
Intraperitoneal dissemination and oncological outcomes
Intra-abdominal or vaginal morcellation as previously described, lead to micro or macro fragments dissemination in the peritoneal cavity.
If this fact is completely depending on morcellation technique alone is also an issue. According to some authors for example, the simple myometrial tissue manipulation during myomectomy could lead to intraabdominal seeding and lympho-vascular invasion of eventual malignant cells from undiagnosed LMS. In cytologic studies, positive washings for myometrial cells were found positive in half patients after myomectomy closures prior to morcellation. Some other studies conducted on morcellation with containment system reported a 9.2% spillage in peritoneal lavage even with intact bags (33,34). It’s not probably possible to reach clear conclusions from these data, as real implication of positive washing is unknown and possible bias exist due to different bag systems used and surgeons’ experience.
Even in the circumstances of benign lesions, consequences as development of endometriosis implants or parasitic myomas are described and a second surgery could be required (35). Parasitic myoma is a myoma completely separated from uterus and receiving blood supply from another structure. A systematic review by Lete et al. revealed in these patients an history of previous myomectomy or hysterectomy in 44% of cases (36). Anyway, other studies demonstrated an incidence of parasitic myomas after uncontained morcellation less than 1% (35,37). Another very rare conditions reported in literature are the peritoneal leiomyomatosis and metastatic leiomyomatosis, that are almost anecdotical situations in which myometrial tissues are found in multiple foci in the abdominal peritoneum, retroperitoneum or in distant organs. Some, but not all, cases seem to be related to previous myomas morcellement (38). Anyway, very small data on these issues are available in literature.
In the event of an occult sarcoma with unprotected morcellation and dissemination, oncological prognosis seems to be decreased. Morcellement should be considered an upstaging and, as previously seen, oncological outcomes strictly correlate with stage at diagnosis. Anyway, uterine LMS has an aggressive biological behaviour per se.
The impact of primary surgery on patients’ survival has been investigated for years. In 2009, Perri et al. retrospectively collected data from women with stage I LMS, underwent previous surgery for presumed fibromas and compared the disease-free survival and overall survival between group A (total abdominal hysterectomy) and group B (any other surgery with “tumour injuries”). Kaplan-Meier curves reveals significantly better results for group A, despite further surgery and oncological therapies (39). According to Park et al. morcellation truly contributes to grave prognosis. They assessed 56 patients with stage I and II uterine LMS, 25 with and 31 without tumour morcellation. In univariate analysis, only tumour morcellation was significantly associated with poorer disease-free survival (P=0.043). Higher stage and tumour morcellation were significantly associated with poorer overall survival in multivariate analysis.
The percentage of patients with abdominal-pelvic dissemination or vaginal recurrence, was significantly greater in patients with previous morcellation (44% vs. 12.9%, P=0.032) (40).
Analogue results are reported in a retrospective study from Norway on patients with uterine sarcoma. The authors identified from the national registries 1,367 women with uterine sarcoma and compared the survival between morcellated and non-morcellated groups. Age-adjusted 10-year uterine sarcoma survival was 32.2% for women treated with morcellation compared with 57.2% for non-morcellated group. All-cause 10-year survival was 32.2% in the morcellated group and 44.1% in the non-morcellated group (25). Gao et al. reported longer 5-year recurrence-free survival and overall survival in uterine sarcoma patients who had not undergone morcellation (43.6% vs. 24.1%; 43.1% vs. 37.8%), but this did not reach statistical significance after multivariate survival analysis (41). Similarly, a retrospective study of 125 patients with LMSs revealed a 3-fold increased risk of death for those who had undergone morcellation. No significant effect was noted for those affected by uterine STUMP or other uterine sarcomas (42).
A recent retrospective publication by Gitas et al. from 3 centers in Germany on 1,683 patients treated with electronical morcellation, found 4 women (0.24%) with unexpected sarcoma (2 LMSs and 2 ESSs). They underwent laparoscopic hysterectomy because all were older than 45 years and 75% had a solitary lesion. In this study, after further treatment, all patients were alive at 5 years of follow-up (24). No unexpected malignancies were observed after laparoscopic myomectomies (probably due to bias selection as patients age).
Several recent studies have directly investigated spread of occult LMS after morcellation within containment systems (43-47). Results are often inconsistent due to small numbers, mostly regarding just surgical outcomes of the technique. Zullo et al. included all randomised controlled trials comparing in-bag extracorporeal manual morcellation versus intracorporeal uncontained power morcellation during laparoscopic myomectomy in premenopausal women, finding no differences in surgical complications or postoperative diagnosis of LMS (43). In another study on 720 patients from India, no case of LMS was found and in-bag versus conventional morcellation were comparable in terms of surgery duration and blood loss (44). Steller et al. reported on 187 patients, with no bag-related complications (45). According to some Authors operative times seem to be slightly prolonged but this is not confirmed using modern system and power morcellator (46). Solid data on oncological safety of in-bag morcellation are missing, probably due the rarity of the event. Rationally this should guarantee no peritoneal dissemination, however, in 2018, Salman and Colleagues described a case-report of a 37 years old patient underwent laparoscopic myomectomy and in-bag morcellation for a suspected myoma histologically revealed as LMS, that recurred after only 5 months (47).
There is little evidence that performance of bilateral salpingo-oophorectomy in premenopausal women improves survival (12,48).
Morcellation injuries
As morcellations have become frequent during surgery, morcellators-related injuries increased, but true rates are difficult to define because of data inconsistency. Probably, the actual frequency is yet unknown and underestimate. Most common complications involve small and large bowel, bladder, ureteral and vascular injuries.
The most comprehensive study currently published in the literature is a systematic review by Milad et al. They published morcellator-related complications from 1993 to 2003. The data was taken from US FDA database. A total of 55 complications were identified: 31 injuries to the small and large bowel, 27 to large blood vessels, 3 to the kidney, 3 to the ureter, 1 to the bladder and 1 to the diaphragm. In six cases, the accidents were fatal. This study concluded that surgeon inexperience and lack of visualization due to collapse of pneumoperitoneum were the common reason for these complications (49).
In 2017, a survey of European Society of Gynaecological Endoscopy (ESGE) members found that direct morcellator injury was a rare event. Despite the low risks, however, also the ESGE board claims that only physicians with adequate training and knowledge should perform these procedures. A total number of 221 LMS was reported among 429,777 minimally invasive surgeries (including hysteroscopic myomectomies) (50,51).
Preoperative evaluation: is possible to define a “high risk” patient?
Preoperative work up has a central role in women with myometrial masses candidate to minimally invasive surgery. Differential diagnosis between benign fibroids and sarcomas is crucial for a better counseling in order to avoid surgical complications related to an unexpected tumour (29,52-54). Although the definitive diagnosis of sarcoma is only histological, several clinical and imaging features have been identified to predict the risk of malignancy (53-55).
Clinical features
Advanced age and postmenopausal status are risk factors for all histological type of sarcoma. The median age of women at LMS diagnosis was 50–56 years (29,55,56). Below the age of 40 a sarcoma in a presumed fibroid is extremely rare. Clinical presentation of uterine sarcomas overlaps with common symptoms associated with uterine fibroids and the preoperative detection rate is low. Presenting symptoms include abnormal uterine bleeding (56%) either in the pre- or postmenopausal period, a palpable pelvic mass (54%), abdominal pain (22%), bloating or abdominal distension and urinary symptoms (52).
Rapid increase in size of a pre-existing myoma is a frequent indication for gynecological oncologist consultation, however is not typical of malignancy, occurring also in benign lesion. Both sarcoma and myoma are responsive to estrogen and gonadotropin-releasing hormone (GnRH) analogue (52,53). Malignancy should be suspected in cases of tumor growth in postmenopausal women who are not on hormone replacement therapy. No growth in 3 months may be reassuring unless caused by GnRH.
Recently, ulipristal acetate (UPA) therapy has been used worldwide for symptomatic uterine myomas demonstrating good results in reducing volume and symptoms (54). Worsening of uterine bleeding or pelvic weight despite medical treatment, were frequently reasons for the patients to choose surgery. Few cases from Italy (2), France (2), Denmark (1) in which postoperative analysis revealed LMS after UPA therapy failure are reported in literature. Obviously, it is not possible to take any solid indication from these scanty data, but ineffective UPA treatment may indicate a population in which uterine LMS is more prevalent and the awareness of this possibility could avoid a delay in the diagnosis and potentially dangerous morcellation (55-58).
Imaging
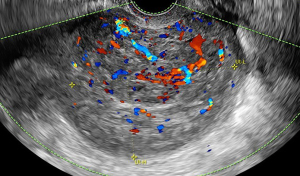
Uterine sarcomas include several histological types so they may display in various morphological patterns. No imaging modality has been shown to be predictive in differentiating between myoma and sarcoma, but some features are more related to malignancy. Transvaginal ultrasound is the first-line imaging tool in evaluation of myometrial pathology (59-62). Morphological Uterus Sonographic Assessment (MUSA) statement provides a consensus on terms, definition and measurement to report myometrial sonographic features, underlying the importance of a homogenous description of the mass (63). As previously described in this number, ultrasound features of uterine sarcomas may be indistinct from those of common fibroids although they may present more frequently as solitary, large size, irregular solid mass often with anechoic area due to necrosis and irregular intralesional vascularisation (64,65). Data on ultrasound prediction of uterine sarcoma are scanty and based on small retrospective series. Recently a retrospective multicentric study describe the clinical and sonographic characteristic of 183 uterine sarcoma reviewed by a consensus meeting according to standard terminology (66). Median age was 56 years and most frequent symptom was abnormal uterine bleeding (but occurring only in almost 50% of women). Uterine sarcomas typically appear as single (80%), solid mass (79.5%), with inhomogeneous echogenicity (77.4%), sometimes with irregular cystic areas (44.6%), only 2.1% with fan shaped shadowing. The vascularisation at color Doppler is moderate or rich (67.9%) (Figure 2).
Magnetic resonance imaging (MRI) is an accurate imaging technique for preoperative selection in women candidate to minimally invasive surgery. T2-weighted sequences, may help evaluating lesion extent and characteristics (29,54,67). Diffusion weighted imaging (DWI) and apparent diffusion coefficient (ADC) values has been reported as selection criteria to stratify low or high-risk masses. Intermediate DWI and low ADC values are correlated with malignancy (68,69).
MRI findings for a suspected LMS are high signals in T2WI and abnormal signals in DWI, high signals in T1WI, undefined borders, high cell density in the mass, suggesting haemorrhage within the mass and an infiltrative growth of mass. In contrast-enhanced images, a mass that shows an early period of heterogeneous, strong contrast effect is considered to be a tumour mass. However, the haemorrhagic necrosis portion of a mass, essential for the diagnosis of LMS, is considered to be an area where contrast effects are missing (70).
The use of CT scan is not supported in evaluation of a myometrial lesion due to the diagnostic superiority of MRI. The use of positron emission tomography (PET) scanning with active metabolite usually fluorodeoxyglucose (FDG) or alphafluorobeta-estradiol (FES) has been reported in preoperative studies of suspected uterine sarcoma, but limited to small series (53,71,72).
Endometrial biopsy
As first diagnostic step an accurate ultrasound evaluation should describe also the distance between uterine mass and endometrial line leading the need of subsequent investigations. Despite imaging data, endometrial sampling is often performed in women with uterine fibroids presenting with abnormal uterine bleeding both in pre and in post menopause to exclude concomitant endometrial pathology (23,29). Preoperative endometrial biopsy found a uterine sarcoma in 3.5%, with a low predictive value reported for uterine sarcomas, as they originate in the myometrium (73). Nevertheless, endometrial sampling is recommended in preoperative work up of uterine mass involving the endometrium, especially when uterine morcellation is likely (29,52,53). The role of endometrial biopsy in asymptomatic patient with uterine fibroids is not clear (74). A good negative predictive value is reported using MRI-guided needle biopsies, no data are found on the possible spread of sarcoma cells by multiple puncturing of the sarcoma (53).
Serum markers
Serum lactate dehydrogenase (LDH) and cancer antigen (CA)-125 have been considered as markers to predict uterine sarcoma (29,75-77). Total LDH and LDH isozyme 3 may be useful in differentiating between sarcoma and myoma: an increment has been reported in prospective series (53,75). CA-125 may be increased in advanced disease, but unfortunately it has low specificity and poor positive predictive value in early stage (29,76). Considering previous cited parameters, Nagai et al. point out scores to predict the risk of sarcoma in a myometrial lesion (29,78,79). Predictive factors include: age over 49, high levels of LDH (>279 U/L), positive MRI and cytological findings. The accuracy, positive predictive value and negative predictive value were 93.7%, 92.3% and 94% respective. Recently an algorithm found that 7 preoperative variables on 32, were associated with increased risk of sarcoma: post-menopausal status, symptom of pressure, post-menopausal bleeding, neutrophil count (>7.5×109), Hb level <11.8 g/dL, endometrial biopsy positive for atypia or neoplasia, mass size >10 cm in radiological imaging (80).
In conclusion preoperative diagnosis of uterine sarcoma remains challenging but the knowledge of clinical presentation and imaging features raise the suspicious index (10,29,81,82). A transvaginal ultrasound performed by an expert sonographer is the first step in preoperative work to define the risk of malignancy of a myometrial mass. Although sonographic imaging may overlap with those of benign or degenerate fibroids, a large, solitary, solid, highly vascularised, heterogeneous myometrial tumor with necrotic changes and absence of calcifications or shadows should raise the suspicion of a sarcoma. A systematic ultrasound evaluation of the uterine cavity together with endometrial biopsy in women with abnormal uterine bleeding is recommended to rule out concomitant endometrial pathology, especially when morcellation is likely. In suspected case an MRI with contrast enhancement and serum LDH (total and isozyme 3) may help to clarify differential diagnosis. A multidisciplinary team discussion of atypical myoma cases prior to minimally invasive surgery can lead to a more appropriate management (Figures 3,4).
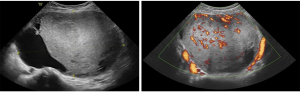
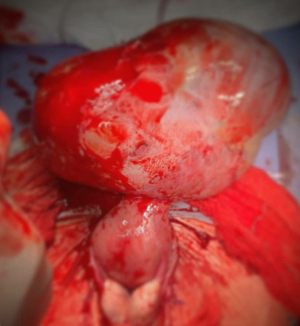
Conclusions
The percentage of women requiring surgery for presumed fibroids is extremely high in every gynaecological institution. Laparoscopic/robotic myomectomy is a feasible surgical technique for those who desire fertility-sparing treatments and if performed by well-trained surgeons, it is safe even including morcellation. The benefits of mini-invasive surgery are well recognized in literature. LMS incidence in the setting of myomectomy/hysterectomy candidates for benign conditions, seems to be lower than reported in 2014 FDA warning. A possible explanation is the selection when regarding myomectomy, of a big number of younger patients (frequently desiring pregnancy), with multiple mesenchymal lesions that show a “typical” aspect on transvaginal ultrasound. In this context, indiscriminate appeal to laparotomy is not reasonable (10,29,81,82).
Nevertheless, in the event of LMS dissemination or non-en bloc dissection, the oncological prognosis seems to be decreased (82), even despite further treatment and adjuvant therapies, and, as said, a preoperative differential diagnosis in myometrial lesions is still challenging.
Literature information are often controversial due bias selection of patients included in the studies with different ages, different myometrial lesions and treated with different surgical approaches.
Ultrasound evaluation in uncertain cases should be performed by an expert gynaecological Sonographer, focusing on number, volume, position, growth, vascularization, necrosis, inhomogeneity, absence of calcifications or shadowing. This diagnostic moment is fundamental not only on oncological bases, but to guide the surgical strategy too. In cases suspect for malignancies MRI could be helpful, even if limits exist because of atypical myomas (69,70).
Postmenopausal patients, especially if irregular bleeding, with single vascularized lesion are more at risk to reveal a malignant tumour and in these cases a laparotomic hysterectomy (or vaginal if feasible) is probably still to be considered. In bag morcellation could be an alternative permitting minimally invasive surgery, but clear data are missing as containment system probably cannot completely prevent cell spread. Anyway, this practice should be rationally implemented, as suggested by the 2020 FDA recommendation.
In the future we should probably define an “high risk” patient among women with myometrial pathology in order to define a restricted group of women who will benefit from avoiding morcellation: future studies should find a modality to stratify the risk of malignancy of a myometrial mass using diagnostic tools and clinical signs. Carefully inspections of morcellated specimens and washing by the pathologists are mandatory.
Patients should be deeply informed about possible surgical strategies including morcellation pros and cons (14,82,83). It could be desirable in every gynaecological unit the establishment of a “myometrial board” including gynaecological Surgeons, expert Sonographer, dedicated Radiologist and Pathologist in order to define multidisciplinary the best strategy for every patient with suspect myometrial mass.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Anna Myriam Perrone and Pierandrea De Iaco) for the series “Uterine Sarcomas” published in Gynecology and Pelvic Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-11/rc
Peer Review File: Available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-11/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-11/coif). The series “Uterine Sarcomas” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril 2007;87:725-36. [Crossref] [PubMed]
- Munro MG, Critchley HO, Broder MS, et al. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet 2011;113:3-13. [Crossref] [PubMed]
- Alessandri F, Lijoi D, Mistrangelo E, et al. Randomized study of laparoscopic versus minilaparotomic myomectomy for uterine myomas. J Minim Invasive Gynecol 2006;13:92-7. [Crossref] [PubMed]
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol 2008;112:387-400. [Crossref] [PubMed]
- Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev 2009;CD003677. [PubMed]
- Bhave Chittawar P, Franik S, Pouwer AW, et al. Minimally invasive surgical techniques versus open myomectomy for uterine fibroids. Cochrane Database Syst Rev 2014;CD004638. [Crossref] [PubMed]
- Roberts ME, Aynardi JT, Chu CS. Uterine leiomyosarcoma: A review of the literature and update on management options. Gynecol Oncol 2018;151:562-72. [Crossref] [PubMed]
- Travaglino A, Raffone A, Santoro A, et al. Prognostic significance of atypical mitotic figures in smooth muscle tumors of uncertain malignant potential (STUMP) of the uterus and uterine adnexa. APMIS 2021;129:165-9. [Crossref] [PubMed]
- Mbatani N, Olawaiye AB, Prat J. Uterine sarcomas. Int J Gynaecol Obstet 2018;143:51-8. [Crossref] [PubMed]
- ACOG Committee Opinion No. 770: Uterine Morcellation for Presumed Leiomyomas. Obstet Gynecol 2019;133:e238-48. Erratum in: Obstet Gynecol 2019 Oct;134(4):883. doi: 10.1097/AOG.0000000000003492. [PubMed]
- Amant F, Coosemans A, Debiec-Rychter M, et al. Clinical management of uterine sarcomas. Lancet Oncol 2009;10:1188-98. [Crossref] [PubMed]
- Kapp DS, Shin JY, Chan JK. Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas: emphasis on impact of lymphadenectomy and oophorectomy. Cancer 2008;112:820-30. [Crossref] [PubMed]
- Abeler VM, Røyne O, Thoresen S, et al. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology 2009;54:355-64. [Crossref] [PubMed]
- The United States Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA Safety Communication. Silver Spring: US Food and Drug Administration; Apr 2014. Available online: https://wayback.archiveit.org/7993/20170722215731/https:/www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm;
- Stentz NC, Cooney LG, Sammel M, et al. Changes in Myomectomy Practice After the U.S. Food and Drug Administration Safety Communication on Power Morcellation. Obstet Gynecol 2017;129:1007-13. [Crossref] [PubMed]
- Mandato VD, Torricelli F, Pirillo D, et al. Impact of the Food and Drug Administration Safety Communication on the Use of Power Morcellator in Daily Clinical Practice: An Italian Survey. J Minim Invasive Gynecol 2016;23:206-14. [Crossref] [PubMed]
- Seidman MA, Oduyebo T, Muto MG, et al. Peritoneal dissemination complicating morcellation of uterine mesenchymal neoplasms. PLoS One 2012;7:e50058. [Crossref] [PubMed]
- Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol 2007;14:453-62. [Crossref] [PubMed]
- Mettler L, Maass N, Abdusattarova K, et al. Frequency of uterine sarcomas in patients admitted for uterine fibroid surgery. J Turk Ger Gynecol Assoc 2017;18:62-6. [Crossref] [PubMed]
- Hartmann KE, Fonnesbeck C, Surawicz T, et al. Management of uterine fibroids. Comparative Effectiveness Review No. 195. AHRQ Publication No. 17(18)-EHC028-EF. Rockville (MD): Agency for Healthcare Research and Quality; 2017. Available online: https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/cer-195-uterine-fibroids-final_0.pdf. Retrieved November 6, 2018.
- Pritts EA. The prevalence of occult leiomyosarcoma in women undergoing presumed fibroid surgery and outcomes after morcellation. Curr Opin Obstet Gynecol 2018;30:81-8. [Crossref] [PubMed]
- Nugent W, Engelke G, Reicke S, et al. Laparoscopic Supracervical Hysterectomy or Myomectomy with Power Morcellation: Risk of Uterine Leiomyosarcomas. A Retrospective Trial Including 35.161 Women in Germany. J Minim Invasive Gynecol 2015;22:S2-3. [Crossref] [PubMed]
- Lieng M, Berner E, Busund B. Risk of morcellation of uterine leiomyosarcomas in laparoscopic supracervical hysterectomy and laparoscopic myomectomy, a retrospective trial including 4791 women. J Minim Invasive Gynecol 2015;22:410-4. [Crossref] [PubMed]
- Gitas G, Alkatout I, Mettler L, et al. Incidence of unexpected uterine malignancies after electromechanical power morcellation: a retrospective multicenter analysis in Germany. Arch Gynecol Obstet 2020;302:447-53. [Crossref] [PubMed]
- Bretthauer M, Goderstad JM, Løberg M, et al. Uterine morcellation and survival in uterine sarcomas. Eur J Cancer 2018;101:62-8. [Crossref] [PubMed]
- Kotani Y, Tobiume T, Fujishima R, et al. Recurrence of uterine myoma after myomectomy: Open myomectomy versus laparoscopic myomectomy. J Obstet Gynaecol Res 2018;44:298-302. [Crossref] [PubMed]
- Rakotomahenina H, Rajaonarison J, Wong L, et al. Myomectomy: technique and current indications. Minerva Ginecol 2017;69:357-69. [PubMed]
- Fava V, Gremeau AS, Pouly JL, et al. Laparoscopic Myomectomy in 10 Steps. J Minim Invasive Gynecol 2019;26:1009-10. [Crossref] [PubMed]
- Halaska MJ, Gracia M, Laky R, et al. Morcellation of the Uterus: Is There Any Place? Curr Oncol Rep 2020;22:68. [Crossref] [PubMed]
- Andan C, Aksin Ş. Culdotomy in laparoscopic myomectomy and its limits. Eur J Obstet Gynecol Reprod Biol 2020;247:49-54. [Crossref] [PubMed]
- Ghezzi F, Casarin J, De Francesco G, et al. Transvaginal contained tissue extraction after laparoscopic myomectomy: a cohort study. BJOG 2018;125:367-73. [Crossref] [PubMed]
- Gil-Gimeno A, Laberge PY, Lemyre M, et al. Morcellation During Total Laparoscopic Hysterectomies: Implications of the Use of a Contained Bag System. J Obstet Gynaecol Can 2020;42:839-45. [Crossref] [PubMed]
- Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol 2016;214:257.e1-257.e6. [Crossref] [PubMed]
- Toubia T, Moulder JK, Schiff LD, et al. Peritoneal Washings After Power Morcellation in Laparoscopic Myomectomy: A Pilot Study. J Minim Invasive Gynecol 2016;23:578-81. [Crossref] [PubMed]
- Tulandi T, Leung A, Jan N. Nonmalignant Sequelae of Unconfined Morcellation at Laparoscopic Hysterectomy or Myomectomy. J Minim Invasive Gynecol 2016;23:331-7. [Crossref] [PubMed]
- Lete I, González J, Ugarte L, et al. Parasitic leiomyomas: a systematic review. Eur J Obstet Gynecol Reprod Biol 2016;203:250-9. [Crossref] [PubMed]
- Van der Meulen JF, Pijnenborg JM, Boomsma CM, et al. Parasitic myoma after laparoscopic morcellation: a systematic review of the literature. BJOG 2016;123:69-75. [Crossref] [PubMed]
- Nguyen D, Maheshwary R, Tran C, et al. Diffuse peritoneal leiomyomatosis status post laparoscopic hysterectomy with power morcellation: A case report with review of literature. Gynecol Oncol Rep 2017;19:59-61. [Crossref] [PubMed]
- Perri T, Korach J, Sadetzki S, et al. Uterine leiomyosarcoma: does the primary surgical procedure matter? Int J Gynecol Cancer 2009;19:257-60. [Crossref] [PubMed]
- Park JY, Park SK, Kim DY, et al. The impact of tumor morcellation during surgery on the prognosis of patients with apparently early uterine leiomyosarcoma. Gynecol Oncol 2011;122:255-9. [Crossref] [PubMed]
- Gao Z, Li L, Meng Y. A Retrospective Analysis of the Impact of Myomectomy on Survival in Uterine Sarcoma. PLoS One 2016;11:e0148050. [Crossref] [PubMed]
- Raspagliesi F, Maltese G, Bogani G, et al. Morcellation worsens survival outcomes in patients with undiagnosed uterine leiomyosarcomas: a retrospective MITO group study. Gynecol Oncol 2017;144:90-5. [Crossref] [PubMed]
- Zullo F, Venturella R, Raffone A, et al. In-bag manual versus uncontained power morcellation for laparoscopic myomectomy. Cochrane Database Syst Rev 2020;5:CD013352. [PubMed]
- Trivedi PH, Trivedi S, Patil S. Laparoscopic In-Bag Morcellation Compared with Conventional Morcellation of Myomas and Uterus with Myomas. J Obstet Gynaecol India 2020;70:69-77. [Crossref] [PubMed]
- Steller C, Cholkeri-Singh A, Sasaki K, et al. Power Morcellation Using a Contained Bag System. JSLS 2017;21:e2016.00095.
- Devassy R, Cezar C, Krentel H, et al. Feasibility of myomatous tissue extraction in laparoscopic surgery by contained in - bag morcellation: A retrospective single arm study. Int J Surg 2019;62:22-7. [Crossref] [PubMed]
- Salman S, Ketenci Gencer F, et al. Unsuspected Diagnosis of Uterine Leiomyosarcoma after Laparoscopic Myomectomy in an Isolated Bag. Case Rep Obstet Gynecol 2018;2018:6342081. [Crossref] [PubMed]
- Zivanovic O, Jacks LM, Iasonos A, et al. A nomogram to predict postresection 5-year overall survival for patients with uterine leiomyosarcoma. Cancer. 2012;118:660-9. [Crossref] [PubMed]
- Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol 2014;21:486-91. [Crossref] [PubMed]
- Tanos V, Brölmann H, DeWilde RL, et al. Survey among ESGE members on leiomyosarcoma morcellation incidence. Gynecol Surg 2017;14:25. [Crossref] [PubMed]
- Tanos V, Berry KE, Frist M, et al. Prevention and Management of Complications in Laparoscopic Myomectomy. Biomed Res Int 2018;2018:8250952. [Crossref] [PubMed]
- Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: Epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol 2017;145:208-16. [Crossref] [PubMed]
- Brölmann H, Tanos V, Grimbizis G, et al. European Society of Gynaecological Endoscopy (ESGE) steering committee on fibroid morcellation. Options on fibroid morcellation: a literature review. Gynecol Surg 2015;12:3-15. [PubMed]
- Garnock-Jones KP, Duggan ST. Ulipristal Acetate: A Review in Symptomatic Uterine Fibroids. Drugs 2017;77:1665-75. [Crossref] [PubMed]
- Modaffari P, D'alonzo M, Garbagnati M, et al. Unexpected uterine leiomyosarcoma in a woman with multiple myomas treated with ulipristal acetate: case report and literature review. Gynecol Endocrinol 2018;34:192-4. [Crossref] [PubMed]
- Kadhel P, Smail M, Borja De Mozota D. Inefficiency of ulipristal acetate on uterus leiomyomas as an additional sign to suspect leiomyosarcoma. J Gynecol Obstet Hum Reprod 2017;46:609-11. [Crossref] [PubMed]
- Santoro A, Angelico G, Arciuolo D, et al. Failure of ulipristal acetate treatment as an indication for uterine malignancy: Two case reports. Medicine (Baltimore) 2018;97:e11532. [Crossref] [PubMed]
- Istre O. Unexpected Uterine Leiomyosarcoma During Laparoscopic Hysterectomy Treated 6 Months with Ulipristal Acetate and Contained Power Morcellation. J Minim Invasive Gynecol 2017;24:198. [Crossref] [PubMed]
- Bogani G, Chiappa V, Ditto A, et al. Morcellation of undiagnosed uterine sarcoma: A critical review. Crit Rev Oncol Hematol 2016;98:302-8. [Crossref] [PubMed]
- Cho HY, Kim K, Kim YB, et al. Differential diagnosis between uterine sarcoma and leiomyoma using preoperative clinical characteristics. J Obstet Gynaecol Res 2016;42:313-8. [Crossref] [PubMed]
- Brohl AS, Li L, Andikyan V, et al. Age-specific risk of unexpected uterine sarcoma following surgery for presumed benign leiomyoma. Oncologist 2015;20:433-9. [Crossref] [PubMed]
- Gaetke-Udager K, McLean K, Sciallis AP, et al. Diagnostic Accuracy of Ultrasound, Contrast-enhanced CT, and Conventional MRI for Differentiating Leiomyoma from Leiomyosarcoma. Acad Radiol 2016;23:1290-7. [Crossref] [PubMed]
- Van den Bosch T, Dueholm M, Leone FP, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol 2015;46:284-98. [Crossref] [PubMed]
- Exacoustos C, Romanini ME, Amadio A, et al. Can gray-scale and color Doppler sonography differentiate between uterine leiomyosarcoma and leiomyoma? J Clin Ultrasound 2007;35:449-57. [Crossref] [PubMed]
- Aviram R, Ochshorn Y, Markovitch O, et al. Uterine sarcomas versus leiomyomas: gray-scale and Doppler sonographic findings. J Clin Ultrasound 2005;33:10-3. [Crossref] [PubMed]
- Ludovisi M, Moro F, Pasciuto T, et al. Imaging in gynecological disease (15): clinicalandultrasound characteristics of uterine sarcoma. Ultrasound Obstet Gynecol 2019;54:676-87. [Crossref] [PubMed]
- Lakhman Y, Veeraraghavan H, Chaim J, et al. Differentiation of Uterine Leiomyosarcoma from Atypical Leiomyoma: Diagnostic Accuracy of Qualitative MR Imaging Features and Feasibility of Texture Analysis. Eur Radiol 2017;27:2903-15. [Crossref] [PubMed]
- Kubik-Huch RA, Weston M, Nougaret S, et al. European Society of Urogenital Radiology (ESUR) Guidelines: MR Imaging of Leiomyomas. Eur Radiol 2018;28:3125-37. [Crossref] [PubMed]
- Tamai K, Koyama T, Saga T, et al. The utility of diffusion-weighted MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur Radiol 2008;18:723-30. [Crossref] [PubMed]
- Suzuki A, Aoki M, Miyagawa C, et al. Differential Diagnosis of Uterine Leiomyoma and Uterine Sarcoma using Magnetic Resonance Images: A Literature Review. Healthcare (Basel) 2019;7:158. [Crossref] [PubMed]
- Umesaki N, Tanaka T, Miyama M, et al. Positron emission tomography with (18) Ffluorodeoxyglucose of uterine sarcoma: a comparison with magnetic resonance imaging and power Doppler imaging. Gynecol Oncol 2001;80:372-7. [Crossref] [PubMed]
- Yoshida Y, Kiyono Y, Tsujikawa T, et al. Additional value of 16α-[18F]fluoro-17β-oestradiol PET for differential diagnosis between uterine sarcoma and leiomyoma in patients with positive or equivocal findings on [18F]fluorodeoxyglucose PET. Eur J Nucl Med Mol Imaging 2011;38:1824-31. [Crossref] [PubMed]
- Hinchcliff EM, Esselen KM, Watkins JC, et al. The Role of Endometrial Biopsy in the Preoperative Detection of Uterine Leiomyosarcoma. J Minim Invasive Gynecol 2016;23:567-72. [Crossref] [PubMed]
- Bansal N, Herzog TJ, Burke W, et al. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol 2008;110:43-8. [Crossref] [PubMed]
- Goto A, Takeuchi S, Sugimura K, et al. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer 2002;12:354-61. [Crossref] [PubMed]
- Juang CM, Yen MS, Horng HC, et al. Potential role of preoperative serum CA125 for the differential diagnosis between uterine leiomyoma and uterine leiomyosarcoma. Eur J Gynaecol Oncol 2006;27:370-4. [PubMed]
- Huang GS, Chiu LG, Gebb JS, et al. Serum CA125 predicts extrauterine disease and survival in uterine carcinosarcoma. Gynecol Oncol 2007;107:513-7. [Crossref] [PubMed]
- Nagai T, Takai Y, Akahori T, et al. Novel uterine sarcoma preoperative diagnosis score predicts the need for surgery in patients presenting with a uterine mass. Springerplus 2014;3:678. [Crossref] [PubMed]
- Nagai T, Takai Y, Akahori T, et al. Highly improved accuracy of the revised PREoperative sarcoma score (rPRESS) in the decision of performing surgery for patients presenting with a uterine mass. Springerplus 2015;4:520. [Crossref] [PubMed]
- Lawlor H, Ward A, Maclean A, et al. Developing a Preoperative Algorithm for the Diagnosis of Uterine Leiomyosarcoma. Diagnostics (Basel) 2020;10:735. [Crossref] [PubMed]
- Parker WH, Kaunitz AM, Pritts EA, et al. U.S. food and drug administration's guidance regarding morcellation of leiomyomas: well-intentioned, but is it harmful for women? Obstet Gynecol 2016;127:18-22. [Crossref] [PubMed]
- Sizzi O, Manganaro L, Rossetti A, et al. Assessing the risk of laparoscopic morcellation of occult uterine sarcomas during hysterectomy and myomectomy: Literature review and the ISGE recommendations. Eur J Obstet Gynecol Reprod Biol 2018;220:30-8. [Crossref] [PubMed]
- Endoscopy British Society of Gynaecological. BSGE Statement on Power Morcellation. Available online: https://www.bsge.org.uk/news/bsge-statement-power-morcellation/
Cite this article as: Rossi M, Solfrini S, Tarsitano F, Rosati F, Facchini C, Antonazzo P. Risk of minimal access surgery in uterine leiomyosarcomas: a narrative review. Gynecol Pelvic Med 2022;5:9.


