
Minimally invasive approach for endometrial cancer: robotic assisted vs. straight stick laparoscopy
Introduction
The standard of care treatment for Endometrial Cancer includes total hysterectomy, bilateral salpingo-oophorectomy and lymph node assessment. Staging procedure via straight stick laparoscopy has been described in the early 1990’s (1) and has 20 years later been established as the gold standard, improving patients’ quality of life, reducing perioperative complications, with comparable oncologic outcomes (2-6). Despite its proven benefits, the adoption of minimally invasive surgery (MIS) has been slow, and laparotomy remained the dominant approach (7,8). Robotic-assisted laparoscopy was introduced when the Da Vinci Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA, USA) was approved by the FDA for gynecologic surgery in 2005. Among the technical advantages of robotic assisted surgery (RAS) over straight stick laparoscopic surgery (LS) are 7 degrees of movement, smaller instruments, improved three-dimensional immersion vision with no fulcrum effect, neutralization of tremor, and control of the camera by the primary surgeon. Potential disadvantages of robotic assisted surgery include lack of haptics and the high acquisition cost. The Laparoscopic Approach to Cervical Cancer (LACC) trial, an international multi-center randomized trial, unexpectedly reported a worse oncologic outcome for the patients with cervical cancer who underwent MIS, and challenged our perception of the safety of MIS, and refocused all of us on the importance of safe oncologic principles in surgery. In this article, we aim to review the advantages and shortcomings of robotic assisted laparoscopy compared to straight stick laparoscopy and the impact of MIS on the treatment of endometrial cancer patients.
Procedure and perioperative outcome
Duration of procedure
Robotic assisted surgery requires a particular setup of the operative room as well as additional time required for the docking process, whereas the technical advantages including better visualization and intuitive manipulation are expected to facilitate and accelerate complex procedures. Therefore, the overall effect of RAS on operative times compared to LS was questioned. In a meta-analysis by Ind et al. including 36 studies and 8,075 patients, studies have shown variable findings, with some retrospective studies reporting longer operating time for RAS by 18.4 min, while a randomized control trial (RCT) found RAS to have a shorter operating time (9). Overall, the total operating theater time was similar (retrospective studies) or even shorter (RCT).
Lymph node assessment and sentinel lymph node mapping
RAS and LS had a higher rate of lymphadenectomy compared to laparotomy for staging in high risk EC, while the median number of lymph nodes extracted or the number of positive nodes did not differ (10). The number of pelvic or paraaortic lymph nodes obtained using RAS and LS was found to be similar in a meta-analysis comparing the two approaches (9). These studies were relevant as benchmarks, although today the value of the number of nodes resected has lost its relevance in view of the introduction of the more targeted sentinel node mapping.
In recent years, sentinel lymph node (SLN) sampling was shown to be a good alternative to lymphadenectomy in endometrial cancer demonstrating good detection rate with high negative predictive value and reduced morbidity (11). Sentinel lymph node procedure in the SENTI-ENDO study was performed by either open or laparoscopic approach and used dual mapping with technetium and patent blue. Detection rate of any SLN for laparoscopic cases was 90% with 65% bilateral mapping (12). In the more recent FIRES study, SLN mapping using indocyanine green (ICG) during RAS, showed successful mapping of 86% with bilateral mapping 52% (13). Sentinel lymph node mapping using ICG was studied in high-risk endometrial cancer undergoing both LS (14) and RAS (14,15) with positive node detection sensitivity of 96% and 98% respectively. A recent Australian study comparing LS and RAS for SLN mapping reported a slightly higher overall detection rate for laparoscopy (97% vs. 88%), with similar bilateral mapping rate (16). The small number of robotic cases (n=33) could explain these findings, given that the learning curve for robotic SLN mapping using indocyanine green was reported to reach a plateau after 27–40 cases (17,18).
Perioperative complications
Numerous studies have compared perioperative outcome between MIS approaches. Average blood loss associated with RAS was reported by Ind et al. to be reduced by 57.7 mLcompared to LS, however this difference was not clinically significant, and transfusion rate and hemoglobin levels did not differ between the groups (9). No significant difference was found for adverse outcomes, including re‐interventions, re‐admissions, post‐operative complications or major post‐operative complications. However, robotic assisted surgery had fewer total complications compared to laparoscopy (RR =0.82).
The rate of conversion of laparoscopy to laparotomy has varied considerably from no conversion (19) to 25.8% (5).Evaluating factors that affect the rate of conversion to laparotomy in LS, morbid obesity [odds ratio (OR), 4.51], suboptimal pelvic examination or enlarged uterus during preoperative evaluation, para-aortic lymphadenectomy, uterine size 250 g or greater, and the presence of extrauterine disease were found to be independent predictors for conversion to laparotomy (20). In a multicenter study, Palomba et al. reported a conversion rate of 13.9% of 512 patients undergoing laparoscopy for EC. After Adjusting for stage and other confounding factors, conversion did not significantly affect recurrence rate or overall survival (21).
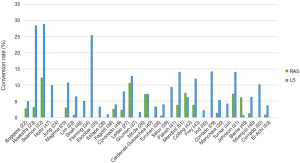
Thanks to its technical advantages, including better ergonomics and more intuitive manipulation, robotic surgery was expected to allow more complicated procedures to be completed using MIS and reduce the need for conversion to laparotomy. Studies comparing conversion rates to laparotomy for LS and RAS are presented in Figure 1. In a randomized controlled trial, Mäenpää et al. 10% conversions were observed in LS vs. none in the RAS group. On the other hand, a population-based study, including 4,034 Laparoscopic surgeries and 6,313 robotic assisted cases showed a similar conversion rate (54). In a meta-analysis by Ind et al. RAS had fewer conversions compared to LS (115 vs. 274) with a relative risk of 0.41 (9).
Several studies, including a meta-analysis, reported a shorter hospital stay following RAS vs. LS (9), however, it was suggested that variability in management protocols among surgeons contributed to this difference. A large retrospective study comparing surgical approach in 3,712 patients with EC, reported that the mean length of stay was similar between RAS and LS, while it was 2.3 days longer for laparotomy. Robotic assisted procedures were associated with fewer early readmissions, but no difference in overall readmission rate (55). In a recent study reporting the initial experience with RAS in New south Wales, Australia, hospital stay was shorter for RAS compared to LS (1.3 vs. 1.8 days) (56).
Studies have shown that the use of opioids analgesics is reduced in RAS compared to laparotomy (57) and LS (58),however, others failed to show a difference (44). No differences could be demonstrated between the two groups for pain scores or post‐operative analgesia usage in a meta-analysis (9). Herniation through trocar incisions has been shown to be associated with higher BMI, though similar rates were found for LS and RAS (59). MIS for EC is involved with a low post-operative mortality rate, which was shown to be similar between LS and RAS in a meta-analysis by Behbehani et al. (60).
Quality of life
Quality of life (QoL) assessment is a complementary measure in evaluating the outcome of a surgical approach. Several randomised trials showed an advantage LS over laparotomy in QoL measures (3,61). In the GOG LAP2 trial, analysis of postoperative QoL showed an advantage for LS in several parameters including physical functioning, less pain, and earlier resumption of physical activity and return to work (4). In contrast, the overall adjusted QoL did not meet the minimally important difference (MID) between the two surgical arms over 6 weeks. Studies that evaluated immediate QoL following RAS have reported a return to baseline 3–5 weeks post surgery (62,63) which suggests an advantage over LS, although it may originate from different analysis methods. Ferguson et al. conducted a multi-center study evaluating QoL and sexual health post RAS, LS, and laparotomy using a series of validated questioners (64). MIS was associated with improved QoL at 3 months and functional well-being at 6 months compared to laparotomy. No difference was found between laparoscopy and RAS. Surgical approach did not have a significant effect on sexual health, although all patients met criteria for sexual dysfunction.
Oncologic outcome and survival
The impact of MIS approach on recurrence and survival in EC has been studied extensively. The LAP2 study included 2,182 patients, of which 31.4% had stage IB and above, and had similar recurrence rates between LS and laparotomy (11.4 vs. 10.2) and similar 5-year overall survival (OS, 89.8%) (5). These findings were further supported by the Laparoscopic Approach to Cancer of the Endometrium (65) randomized study which demonstrated a similar 4.5 DFS (0.3% difference favoring laparoscopy) and OS between LS and laparotomy in 760 patients (2).
In addition to these randomized controlled studies, some studies have presented discrepant findings. Song et al. compared RAS vs. laparotomy in 179 patients with high-intermediate risk EC (66), and found a recurrence rate of 5.9% and a 5-year DFS of 91.8% in the RAS group whereas surprisingly none of the laparotomy group patients recurred. Similar results were found with a HR of 0.9 comparing MIS to open approach in high-risk EC (67), and in a retrospective analysis of the National Cancer Database (68).On the other hand, Monterossi et al. reported an increase in recurrence rate after laparotomy compared to MIS in patients with type II endometrial cancer (31.7% vs. 17.7%) (69). In addition, an observational study including 419 patients with high-intermediate risk EC who underwent staging including pelvic and paraaortic lymphadenectomy found that LS was associated with improved overall survival compared to open surgery on multivariate analysis, but recurrence rate and recurrence free survival (RFS) were similar (70).
Data analysis of the Danish population between 2005 and 2015 has shown an improved OS since the introduction of robotic assisted surgery (HR of 1.22). Following the incorporation of RAS in the management of EC, laparotomy procedures were associated with decreased OS compared to RAS and LS, whereas no significance difference between MIS approaches was reported (71). In a retrospective study by Cardenas-Goicoechea et al. including 415 women, no significant difference was found between RAS and LS in recurrence rate (14.8% vs. 12.1%), 3-year disease free survival (DFS, 83.3% vs. 88.4%) and 3-year OS (93.3% vs. 93.6%) (40). Comparison of single port laparoscopy to LS and RAS did not find difference in progression free survival (PFS) or OS (72). A more recent study summarizing 10 years of robotic experience in a single institution, did not find differences in 5-year DFS or OS between MIS to laparotomy as well as between RAS and LS (73).
Port site recurrence has always been evaluated following MIS in gynecologic oncology (74). The LAP2 study reported a presumed trocar recurrence rate of 0.24% (5), and Barraez et al. have reported a low rate for port site metastasis (0.9%) in 438 patients undergoing RAS for EC (75).
General effect on rate of MIS surgery
When examining the impact of robotic surgery on the surgical management of endometrial cancer, a broader view can be used by examining the effect of introducing RAS on the rate of MIS. While laparoscopic approach has been available for almost three decades, the rate of LS increased very gradually, benefitting around 15% of eligible cases (76,77). Early reports have shown that by introducing RAS practice into the care of endometrial cancer, the overall MIS rate was increased by 34.2–81% and was associated with shortened hospital stay and reduced complication rates (50,78).
Database analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) showed that although MIS was associated with increased operating times, it decreased hospital stay by 2.4 days and was associated with a significant decrease in postoperative complications (8). In a follow-up study based on the ACS-NSQIP database by Casarin et al., laparotomy increased odds ratios for major complications (OR=2.4), perioperative complications and perioperative death (OR=3.8). Between 2008 and 2014 an increase in overall MIS rate from 24.2% to 71.4% was accompanied by a decrease in 30-day morbidity (79). An updated analysis published in 2018 distinguished between MIS approaches. The implementation of RAS in the United States has resulted in an absolute 47.3% increase in RAS for endometrial cancer between 2008 and 2015 (9.48% to 56.82%), while the rate of open surgery has declined by 42.37% (70.45% to 28.08%) and a 4.28% absolute reduction in LS was observed (18.11 to 13.83%) (80). These trends were associated with reduced rate of perioperative morbidity without significant increase in cost compared to open surgery. The effect of RAS on surgical management of EC was not limited to the United States and Canada. In a recent Danish nationwide prospective cohort study including 5,654 with early-stage EC, the introduction of robotic surgery has resulted in a significant increase in the rate of MIS from 3% to 95% and was associated with a reduction in severe complication rate (81).
Special considerations
Obesity
The management of EC is further challenged by the fact that up to 80% of patient are obese and 19% to 36% are morbidly obese and have a higher rate of associated co-morbidities.
Obesity has been associated with increased perioperative morbidity including venous thromboembolism and surgical wound complications, mainly after open surgical staging (82,83). Challenges associated with surgical staging using MIS approach in obese patients include reduced exposure in addition to difficult ventilation and possible cardiovascular compromise, secondary to increased abdominal pressure combined with steep Trendelenburg position. Several observational studies evaluating the laparoscopic approach for obese patients did not show a difference in surgical outcomes or perioperative complications when compared to non-obese patients (84-86).
Robotic surgery has been shown to be safe in surgical staging of obese patients with endometrial cancer. Retrospective studies evaluating RAS showed that increasing BMI did not affect conversion rate, lymph node dissection rate or yield (87) or postoperative complications (87,88). Compared to laparotomy, RAS had a lower rate of postoperative complications (17.7% vs. 44%) and shorter length of stay (2 vs. 4 days) in patients with BMI >35 kg/m2 (89). Evaluating MIS approach in obese and morbidly obese patients, RAS was associated with shorter operative time, decreased blood loss, and shorter hospital stay compared to LS (90). Table 1 (51-53,89-99) summarizes studies comparing RAS with open surgery and/or LS. In a comparative study evaluating 1,087 morbidly obese EC patients, open surgery was associated with increased blood transfusion rate and longer hospital stay compared to LS and RAS (99). In a multi-institutional study comparing RAS and LS including 655 obese and extremely obese patients, a lower conversion rate and reduction in hospital length of stay were reported in the RAS arm. Estimated blood loss was higher and operating time was longer in the RAS group, which could possibly be explained by a higher rate of pelvic lymphadenectomy in the RAS group compared to the LS group (43% vs. 19.7%) (52). A meta-analysis including 10,800 obese patients showed slightly higher conversion rates for LS compared to RAS in patients with BMI ≥30 (6.5% vs. 5.5%) or BMI ≥40 (7% vs. 3.8%). The most common cause for conversion was insufficient exposure, however, the 31% conversion in LS were due to intolerance of Trendelenburg position vs. 6% of RAS (100).
Table 1
| Study | Surgical approach | BMI criteria (kg/m2) | N | Operative time (min) | EBL (mL) | Conversion to open surgery (%) | Post-op complications (%) | Hospital stay (d) | |
|---|---|---|---|---|---|---|---|---|---|
| Wound related | Other | ||||||||
| Bernardini et al. (89) | OS/RS | >35 | 41/45 | 165/270# | 300/200# | –/8.9 | 19.5/4.4# | 44/17.7^,# | 4/2# |
| Borgfeldt et al. (91) | OS/RS | ≥35 | 28/79 | 193/201 | 427/100 # | NR | NR | 6.1/2.4# | |
| Fornalik (92) | OS/RS | ≥40 | 35/76 | 126/203# | 500/150# | –/0 | 3/1.3 | 29/15 | 5/1# |
| Hinshaw et al. (93) | OS/RS | ≥35 | 80/56 | 200/212 | 338/150# | –/5.4 | 7/2 | 28/9# | 4/1# |
| ≥40 | 52/31 | 225/210 | 488/235# | 4/2 | 17/4# | 4/1# | |||
| Leitao et al. (94) | OS/MIS | ≥40 | 299/125 | 170/191# | 250/125# | 10.5LS/3.4RS | 27/6# | 36/15^,# | 5/1# |
| Nevadunsky et al. (95) | OS/RS | ≥30 | 43/66 | 134/204# | 193/83# | –/9.7 | 20/0# | 4/4 | 3.8/1.3# |
| Seamonet al. (96) | OS/RS | ≥30 | 191/109 | 143/228# | 394/109# | –/15.6 | 17/2# | 27/11# | 4/2# |
| Subramaniam et al. (97) | OS/RS | ≥30 | 104/73 | 246/138# | 409/96# | –/11 | 20.2/4.1# | 29.8/9.6# | 5.1/2.7# |
| Tang et al. (98) | OS/RS | ≥30 | 110/129 | 128/188# | 292/160# | –/10.9 | 32.7/13.9#; 0/6†,# | 36.4/13.2# | 4.1/1.5# |
| Chan et al. (99) | OS/LS/RS | ≥40 | 567/98/422 | NR | NR | NR | 23/13/8# | 4/1/1# | |
| Corrado et al. (52) |
LS/RS | 30–34.9 | 232/130 | 115/176# | 50/100# | 2.2/1.5 | 5.2/6.2 (E)* | 2.2/3.8 (L)* | 3/3 |
| 35–39.9 | 98/61 | 121/170# | 50/100 | 6.1/0# | 4.1/6.6 | 5.1/0 | 3/3 | ||
| 40–49.9 | 62/44 | 110/142# | 50/80# | 1.6/0 | 4.8/6.8 | 3.2/2.3 | 3/3 | ||
| ≥50 | 14/14 | 157/170 | 50/75 | 0/0 | 21.4/14.3 | 0/7.1 | 4/3 | ||
| El-Achiet al. (53) | LS/RS | ≥40 | 33/31 | 196/215 | 98/44# | 0/0 | NR | 1/1 | |
| Gehrig et al. (90) | LS/RS | 30–39.9 | 25/36 | 215/189**,# | 150/50**,# | 7/0 | 24/13.9 | 1.3/1.0**,# | |
| ≥40 | 7/13 | 14/0 | 14.2/7.6 | ||||||
| Mendivil et al. (51) | OS/LS/RS | >40 | 24/16/13 | 81/109/167# | 250/175/100# | –/6.3/7.7 | 16.6/6.3/15.3 | 4/2/2# | |
OS, open surgery; RS, robotic assisted surgery; BMI, body mass index; MIS, minimally invasive surgery; LS, straight stick laparoscopy; y, years; min, minutes; EBL, estimated blood loss; ml, millilitres; d, days; NR, not reported; ^, overall complications rate; #P<0.05; †, vaginal cuff wound complications; *, complications reported as early (E) vs. late (L); **, all BMI categories were reported together.
Elderly patients
Surgical intervention in older patients is affected by the fact that they tend to be frailer and have more comorbidities. For these reasons, this population is expected to benefit greatly from the advantages of MIS. However, surgeons and anesthesiologists refrained from performing MIS in these patients due to fear from complications associated with deep Trendelenburg position. Straight stick laparoscopy was shown to be feasible, however, in patients older than 65 years it was associated with longer operating time and higher rate of transfusion compared to laparotomy in addition to a high conversion rate to laparotomy (22.4%) (101).In a retrospective analysis of patients from Gynecologic Oncology Group LAP2, benefits of LS over laparotomy were more evident in patients over the age of 60, and included reduced rate of postoperative pneumonia, thromboembolism and ileus in addition to shorter hospital stay (102).
RAS was associated with reduced perioperative complications, reduced blood loss and shortened hospital stay when compared to laparotomy (103-106). Several studies have reported MIS outcomes in elderly patients (Table 2) (103-116). Focusing on the effect of age in robotic assisted surgery, studies have reported that perioperative complication rate associated with lymphadenectomy procedure was not significantly altered when a cut-off age of 70 or 75 years was used (116,117). A Healthcare Cost and Utilization Project National Inpatient Sample (HCUP-NIS) database analysis found that age >65 was associated with similar intraoperative complication rate, but higher rates of perioperative (8.3% vs. 5.2%), medical complications (12.3% vs. 6.7%) and longer hospital stay (110) after laparotomy. Among patients above the age of 80 undergoing RAS, a multi-institutional study found no difference in operative outcomes such as operative time, conversion rate or blood loss compared to younger patients. The rate of intraoperative complications did not differ, although there was a higher rate of postoperative complications (33% vs. 13%) (112). Lau et al. reported a similar minor complications rate for patients older than 80 years compared to patients younger than 80, and they resumed activities quicker than younger patients (115). Perioperative complication rate including vascular, urinary and transfusion rate were lower in LS and RAS compared to laparotomy in patients younger than 75 years, whereas in older patients, complication rate did not differ between the surgical approaches. This could be associated with less staging procedures in elderly patients (age >75) (111). Hospital stay was shorter for RAS and LS independent of age.
Table 2
| Study | Surgical approach | Age group (y) | N | EBL (mL) | Intra-op complications (%) | Post-op complications (%) | Hospital stay (d) | ||
|---|---|---|---|---|---|---|---|---|---|
| Minor | Major | Overall | |||||||
| Scribner et al. (107) | OS/LS | 65≤ | 45/67 | 336/298 | 0/7.5 | NR | NR | 57.9/12# | 5.6/3# |
| Bijen et al. (108) | OS/LS | 70≤ | 23/38 | NR | 4.3/5.3 | NR | NR | 17.4/23.7 | NR |
| Bogani et al. (109) | OS/LS | 75≤ | 66/59 | 175/100# | 2/3 | NS | NR | 3/14 | 6/2# |
| Lavoue et al. (104) | OS/RS | 70≤ | 50/113 | 334/75# | 10/6 | 60/17# | 6/4 | NR | 8/3.1# |
| Doo et al. (103) | OS/RS | 65≤ | 47/26 | 235/131# | 14.9/3.8 | 29.8/3.8s,# | 29.8/19.2m | NR | 4.4/2.2# |
| Guy et al. (110) | OS/RS | 65≤ | 5,914/1,228 | NR | 4.1/5.9# | 20.5/8.3s# | 23.3/12.3m,# | NR | 5.1/2# |
| Backes et al. (105) | OS/RS | 70≤ | 93/89 | 300/75# | NR | NR | NR | 94/24† | 4/1# |
| Bourgin et al. (111) | OS/LS/RS | 75≤ | 26/27/16 | NR | 7.6/0/0 | 19.2/3.7/6.2 | 3.8/3.7/0 | NR | 10.7/7.2/4.5# |
| Lindfors et al. (106) | OS/RS | 70≤ | 137/141 | 381/47# | 1/4 | 22/10 | 5/6 | NR | 6.3/2.5# |
| Lowe et al. (112) | RS | <80/80≤ | 395/27 | 50/50 | 5.1/7.4 | NR | NR | 13/33# | 1/1 |
| Siesto et al. (113) | LS | ≤65/65< | 60/48 | 100/100 | 1.7/4.2 | NR | NR | 23.4/25 | 2/2 |
| Frey et al. (114) | LS | <65/65≤ | 36/31 | 166/165 | NR | NR | NR | 12/0 | 1.7/3# |
| RS | <65/65≤ | 25/17 | 218/147 | NR | NR | NR | 2.8/5.6 | 3.5/1.8 | |
| Doo et al. (103) | RS | <65/65≤ | 72/26 | 83/131 | 2.8/3.8 | 2.8/3.8 | 2.8/19.2# | NR | 1.3/2.2# |
| Guy et al. (110) | RS | <65/65≤ | 1,574/1,228 | NR | 6.8/5.9 | 5.2/8.3# | 6.7/12.3# | NR | 1.7/2# |
| Zeng et al. (115) | RS | <70/70-80/80< | 197/75/31 | 78/69/88 | 0.5/0/3 | 16/12/19 | 0/1/10# | NR | 1.6/1.4/5.2# |
| Bourgin et al. (111) | LS | <75/75≤ | 127/27 | NR | 14.9/0# | 11.8/3.7 | 3.1/3.7 | NR | 5.2/7.2# |
| RS | <75/75≤ | 75/16 | NR | 5.3/0 | 8/6.2 | 2.6/0 | NR | 3.7/4.5 | |
| Hotton et al. (116) | RS | <70/70≤ | 86/62 | NR | 2.3/3.2 | NS | NS | 10.5/12.9 | 6.5/6.5 |
OS, open surgery; LS, straight stick laparoscopy; RS, robotic assisted surgery; y, years; min, minutes; EBL, estimated blood loss; d, days; NR, not reported; #P<0.05; s, surgical complications; m, medical complications; †, total complication events.
Ergonomics
Long operating hours is a keystone of gynecologic oncology surgeries. It leads to mental and physical strains on the surgeons, which have an accumulative effect, and at best cannot be beneficial for the patient undergoing surgery. One of the potential advantages of introducing robotic assisted surgery to the field of gynecologic oncology was alleviating the strain associated with prolonged surgery. A prospective French study of 88 robotic and 82 straight stick laparoscopy cases with a minimal duration of one hour evaluated 24 surgeons. The physical discomfort during LS was significantly higher, and the subjective pain score increased significantly during the procedure compared RAS. Concerning the mental demand, the overall workload and performance were significantly greater during the LS compared to the RAS. For young surgeons, the overall workload, effort, mental and physical demands were greater during LS, while for experienced surgeons only the physical demand was increased (118). In a review of ergonomics of gynecological surgeries, the prevalence of work-related musculoskeletal disorders (WMSD) was reported to be the lowest with RAS. The review also reported that RAS was characterized by more freedom, better motion scaling, tremor reduction and less need for arcing maneuvers. Finger pain and eye strain are more common with RAS than other approaches (119).
A survey of 236 of AAGL (American Association of Gynecologic Laparoscopists) affiliated surgeons asked about the physical demands of surgery and how they were affected by RAS, and showed that RAS helped decreasing surgeons’ eye strain, but did not improve post-procedure neck stiffness or finger pain. Surgeons with high RAS volume noticed more pronounced effects, reporting fewer physical demands with RAS (120).
Learning curve
The adoption of any novel surgical approach is accompanied by a period of gaining experience in which a learning curve can be observed. The learning curve for performing surgical staging in EC using the laparoscopic approach was suggested to be between 25–30, mainly due to a high level of experience needed for laparoscopic lymphadenectomy (121-123). In vitro, surgeons who had little MIS experience showed faster improvement in performance of dexterity tasks using RAS compared to laparoscopy. Among experienced surgeons, however, the learning curve was similar between RAS and LS (124). Studies that have evaluated in-vivo learning curve for robotic surgery have reported that the progress curve plateaued after 9–20 cases (32,33,112,125-128), while a large series by Leitao et al. has reported further improvement until completion of 40 cases (27). Compared to laparoscopy, RAS was found to have a shorter learning curve when performing hysterectomy together with pelvic and para-aortic lymphadenectomy (33,126).
Cost effectiveness
Even though straight stick laparoscopy is associated with higher intra-operative instrument costs, it has been shown to reduce total healthcare associated costs when compared to laparotomy due to shorter hospital stays and lower complications rate (65). Since RAS is associated with a higher instrument cost due to the price of the platform, the maintenance, and its related accessories, its cost-effectiveness compared to LS has been questioned. Studies have evaluated cost-effectiveness by measuring just direct costs of surgery and perioperative complications, while others include indirect costs such as the need for rehabilitation, utilization of primary care services and time off the labor market.
It was hypothesized that robotic surgeries will decrease the treatment costs of EC as a result of shorter hospital stays and lower complication rates. A number of studies have shown comparable outcomes between laparoscopic surgeries and RAS with higher costs using RAS (38,54,129-132), and other studies have shown financial advantage to RAS over open surgery (133,134). An early study showed no benefit of RAS in obese women with EC (99), and a meta-analysis reported an increased total surgery cost of RAS by $1,869 (9). However, more recent studies showed improved treatment costs when utilizing RAS in elderly and obese women with EC and hyperplasia (83,94,106).
When analysing the economic impact of Robotic Assisted surgery for EC, one should take into account the overall effect of introducing RAS has on the rate of laparotomies performed. Leitao et al. reported a higher total amortized cost for RAS by $3,175 when compared to LS for newly diagnosed EC. However, a model incorporating the effect of the reduction in open surgery has neutralized the cost difference (132). A study that reviewed patient charts admitted to the gynecologic oncology ward one year before and 5 years following the introduction of RAS to a tertiary care hospital, showed better utilization of hospital resources with the introduction of RAS (79). More patients were admitted and operated on, while hospital stays were shorter resulting in a better turn-over and increased capacity. This turn-over helped adapting more complex cases in the inpatient ward, cases that required advanced medical care. Another benefit was the reduction in the cost of admission by almost a half with the introduction of RAS ($9,827 vs. $4,058) (135). An updated analysis reported that robotic assisted surgery was increased from 15% to 94%, and was associated with a $3.5 million saving during the course of 15 years (136). Korsholm et al. extracted data from a Danish national registry to study the long-term consequences of a nationwide introduction of RAS in treating early-stage EC and cost for evaluated for a period of 12 months before and after surgery. Records analysis of 4,133 patients showed that following the introduction of RAS the long-term health costs per patient increased by $7,309 (130). Of note, despite an increase in the rate of MIS from 22% to 72%, there was no reduction in bed days after adjusting for patients’ characteristics and surgical year, which could explain the high relative cost. In their database analysis, Casarin et al. reported that RAS was associated with a 13.5% reduction in 30-day perioperative morbidity and similar 30-day (US $12,200 vs. 12,018) perioperative total cost compared to laparotomy (80).
Since many of the early studies regarding cost-effectiveness analysed the first years’ experience with RAS, it was questioned whether further improvement of the technique would lower the associated costs of RAS. Avondstondt et al. have indeed reported a 15% reduction in cost of RAS mainly due to reduction in operative times (137). In addition to cost reduction associated with improved experience, increased competition in the field of computer assisted minimal invasive surgery is expected to reduce platform and instrument costs.
Future developments
The field of robotic assisted surgery was dominated by the da Vinci platforms. The gradual expiry of the patents together with development of competing platforms is expected to reshape the landscape of robotic assisted surgery, increase diversity and reduce costs (138,139). By introducing a computer interface into the operating theater, robotic assisted surgery is the stepping-stone towards high-tech surgery. Three-dimensional reconstruction techniques based on preoperative images are already being utilized for intraoperative navigation (140,141) and the use of augmented reality is bound to expand. Artificial intelligence using machine learning, allows the assessment and integration of large volume of data in order to develop surgical skills, improve surgical procedure planning and predict surgical outcomes (142-145). In addition, the possibility of remote control could serve as grounds for the development of tele-surgery (146).
Conclusions
Safety and oncologic outcomes remain the cornerstone of gynecologic oncology surgery. Following the LACC trial, the oncologic outcome of MIS approach for the management of cervix cancer was brought up for discussion, however, in endometrial cancer, MIS approach has been shown by numerous retrospective, prospective, as well as randomized trials to have major perioperative benefits compared to laparotomy, along with equivalent oncologic outcome. Because of the improved dexterity and vision, when compared to straight stick laparoscopy, robotic assisted surgery appears to be associated with reduced perioperative complications, lower conversion to laparotomy and reduced hospital stay, while accompanied by longer operative time and higher cost when directly compared to laparoscopy. The advantages of RAS are sustained in obese and elderly patients. It is fundamental to remember that both straight stick laparoscopy and Robotics are MIS approaches, just with different tools, and the overarching purpose is to allow as many the patients as possible to benefit from MIS rather than open surgery. In rare centers with high proportion of straight stick laparoscopy that are able to maintain a 70–80% rate of minimal invasive surgery for endometrial cancer, there is little added value at present to adopt robotics. Thus, the greatest impact of Robotic Assisted Surgery was to allow the shift toward MIS approach in centers where laparoscopy failed to considerably reduce the rate of laparotomy. In addition, the robotic platform with its stable controlled environment allows for integration of technological developments by incorporating the computer interface between the surgeon and the patient, leading to implementation of digital analysis and artificial intelligence in gynecologic surgery, that will lead to entire new paradigms in surgery.
Acknowledgments
Funding: Dr. Raban was supported by a grant from the Israel Cancer Research Fund. This work was supported in part by the Gloria's Girl Fund, and the Susan & Jonathan Wener Fund.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Federico Ferrari) for the series “Surgical Approaches for Gynecologic Cancers” published in Gynecology and Pelvic Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-1/coif). The series “Surgical Approaches for Gynecologic Cancers” was commissioned by the editorial office without any funding or sponsorship. WHG serves as an unpaid editorial board member of Gynecology and Pelvic Medicine from June 2020 to May 2022. He reports personal fees from Minogue Medical and Intuitive Surgical, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Childers JM, Hatch KD, Tran AN, et al. Laparoscopic para-aortic lymphadenectomy in gynecologic malignancies. Obstet Gynecol 1993;82:741-7. [PubMed]
- Janda M, Gebski V, Davies LC, et al. Effect of Total Laparoscopic Hysterectomy vs Total Abdominal Hysterectomy on Disease-Free Survival Among Women With Stage I Endometrial Cancer: A Randomized Clinical Trial. JAMA 2017;317:1224-33. [Crossref] [PubMed]
- Janda M, Gebski V, Brand A, et al. Quality of life after total laparoscopic hysterectomy versus total abdominal hysterectomy for stage I endometrial cancer (LACE): a randomised trial. Lancet Oncol 2010;11:772-80. [Crossref] [PubMed]
- Kornblith AB, Huang HQ, Walker JL, et al. Quality of life of patients with endometrial cancer undergoing laparoscopic international federation of gynecology and obstetrics staging compared with laparotomy: a Gynecologic Oncology Group study. J Clin Oncol 2009;27:5337-42. [Crossref] [PubMed]
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol 2009;27:5331-6. [Crossref] [PubMed]
- Zullo F, Falbo A, Palomba S. Safety of laparoscopy vs laparotomy in the surgical staging of endometrial cancer: a systematic review and metaanalysis of randomized controlled trials. Am J Obstet Gynecol 2012;207:94-100. [Crossref] [PubMed]
- Fader AN, Weise RM, Sinno AK, et al. Utilization of Minimally Invasive Surgery in Endometrial Cancer Care: A Quality and Cost Disparity. Obstet Gynecol 2016;127:91-100. [Crossref] [PubMed]
- Scalici J, Laughlin BB, Finan MA, et al. The trend towards minimally invasive surgery (MIS) for endometrial cancer: an ACS-NSQIP evaluation of surgical outcomes. Gynecol Oncol 2015;136:512-5. [Crossref] [PubMed]
- Ind T, Laios A, Hacking M, et al. A comparison of operative outcomes between standard and robotic laparoscopic surgery for endometrial cancer: A systematic review and meta-analysis. Int J Med Robot 2017;13:e1851. [Crossref] [PubMed]
- Pulman KJ, Dason ES, Philp L, et al. Comparison of three surgical approaches for staging lymphadenectomy in high-risk endometrial cancer. Int J Gynaecol Obstet 2017;136:315-9. [Crossref] [PubMed]
- Holloway RW, Abu-Rustum NR, Backes FJ, et al. Sentinel lymph node mapping and staging in endometrial cancer: A Society of Gynecologic Oncology literature review with consensus recommendations. Gynecol Oncol 2017;146:405-15. [Crossref] [PubMed]
- Ballester M, Dubernard G, Lecuru F, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTI-ENDO). Lancet Oncol 2011;12:469-76. [Crossref] [PubMed]
- Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol 2017;18:384-92. [Crossref] [PubMed]
- Cusimano MC, Vicus D, Pulman K, et al. Assessment of Sentinel Lymph Node Biopsy vs Lymphadenectomy for Intermediate- and High-Grade Endometrial Cancer Staging. JAMA Surg 2021;156:157-64. [Crossref] [PubMed]
- Persson J, Salehi S, Bollino M, et al. Pelvic Sentinel lymph node detection in High-Risk Endometrial Cancer (SHREC-trial)-the final step towards a paradigm shift in surgical staging. Eur J Cancer 2019;116:77-85. [Crossref] [PubMed]
- Chaowawanit W, Campbell V, Wilson E, et al. Comparison between laparoscopic and robotic surgery for sentinel lymph node mapping in endometrial cancer using indocyanine green and near infra-red fluorescence imaging. J Obstet Gynaecol 2020;1-5: Epub ahead of print. [Crossref] [PubMed]
- Tucker K, Staley SA, Gehrig PA, et al. Defining the learning curve for successful staging with sentinel lymph node biopsy for endometrial cancer among surgeons at an academic institution. Int J Gynecol Cancer 2020;30:346-51. [Crossref] [PubMed]
- Kim S, Ryu KJ, Min KJ, et al. Learning curve for sentinel lymph node mapping in gynecologic malignancies. J Surg Oncol 2020;121:599-604. [Crossref] [PubMed]
- Zorlu CG, Simsek T, Ari ES. Laparoscopy or laparotomy for the management of endometrial cancer. JSLS 2005;9:442-6. [PubMed]
- Matsuo K, Jung CE, Hom MS, et al. Predictive Factor of Conversion to Laparotomy in Minimally Invasive Surgical Staging for Endometrial Cancer. Int J Gynecol Cancer 2016;26:290-300. [Crossref] [PubMed]
- Palomba S, Ghezzi F, Falbo A, et al. Conversion in endometrial cancer patients scheduled for laparoscopic staging: a large multicenter analysis: conversions and endometrial cancer. Surg Endosc 2014;28:3200-9. [Crossref] [PubMed]
- Boggess JF, Gehrig PA, Cantrell L, et al. A comparative study of 3 surgical methods for hysterectomy with staging for endometrial cancer: robotic assistance, laparoscopy, laparotomy. Am J Obstet Gynecol 2008;199:360.e1-9. [Crossref] [PubMed]
- Hoekstra AV, Jairam-Thodla A, Rademaker A, et al. The impact of robotics on practice management of endometrial cancer: transitioning from traditional surgery. Int J Med Robot 2009;5:392-7. [Crossref] [PubMed]
- Jung YW, Lee DW, Kim SW, et al. Robot-assisted staging using three robotic arms for endometrial cancer: comparison to laparoscopy and laparotomy at a single institution. J Surg Oncol 2010;101:116-21. [Crossref] [PubMed]
- Magrina JF, Zanagnolo V, Giles D, et al. Robotic surgery for endometrial cancer: comparison of perioperative outcomes and recurrence with laparoscopy, vaginal/laparoscopy and laparotomy. Eur J Gynaecol Oncol 2011;32:476-80. [PubMed]
- Estape R, Lambrou N, Estape E, et al. Robotic-assisted total laparoscopic hysterectomy and staging for the treatment of endometrial cancer: a comparison with conventional laparoscopy and abdominal approaches. J Robot Surg 2012;6:199-205. [Crossref] [PubMed]
- Leitao MM Jr, Briscoe G, Santos K, et al. Introduction of a computer-based surgical platform in the surgical care of patients with newly diagnosed uterine cancer: outcomes and impact on approach. Gynecol Oncol 2012;125:394-9. [Crossref] [PubMed]
- Nicole N, Rachel C, Michael M, et al. Robotic Assisted, Total Laparoscopic, and Total Abdominal Hysterectomy for Management of Uterine Cancer. J Cancer Ther 2012;3:162-6. [Crossref] [PubMed]
- Corrado G, Cutillo G, Pomati G, et al. Surgical and oncological outcome of robotic surgery compared to laparoscopic and abdominal surgery in the management of endometrial cancer. Eur J Surg Oncol 2015;41:1074-81. [Crossref] [PubMed]
- Manchana T, Puangsricharoen P, Sirisabya N, et al. Comparison of Perioperative and Oncologic Outcomes with Laparotomy, and Laparoscopic or Robotic Surgery for Women with Endometrial Cancer. Asian Pac J Cancer Prev 2015;16:5483-8. [Crossref] [PubMed]
- Johnson L, Bunn WD, Nguyen L, et al. Clinical comparison of robotic, laparoscopic, and open hysterectomy procedures for endometrial cancer patients. J Robot Surg 2017;11:291-7. [Crossref] [PubMed]
- Seamon LG, Fowler JM, Richardson DL, et al. A detailed analysis of the learning curve: robotic hysterectomy and pelvic-aortic lymphadenectomy for endometrial cancer. Gynecol Oncol 2009;114:162-7. [Crossref] [PubMed]
- Lim PC, Kang E, Park DH. A comparative detail analysis of the learning curve and surgical outcome for robotic hysterectomy with lymphadenectomy versus laparoscopic hysterectomy with lymphadenectomy in treatment of endometrial cancer: a case-matched controlled study of the first one hundred twenty two patients. Gynecol Oncol 2011;120:413-8. [Crossref] [PubMed]
- Fleming ND, Axtell AE, Lentz SE. Operative and anesthetic outcomes in endometrial cancer staging via three minimally invasive methods. J Robot Surg 2012;6:337-44. [Crossref] [PubMed]
- Escobar PF, Frumovitz M, Soliman PT, et al. Comparison of single-port laparoscopy, standard laparoscopy, and robotic surgery in patients with endometrial cancer. Ann Surg Oncol 2012;19:1583-8. [Crossref] [PubMed]
- Fagotti A, Gagliardi ML, Fanfani F, et al. Perioperative outcomes of total laparoendoscopic single-site hysterectomy versus total robotic hysterectomy in endometrial cancer patients: a multicentre study. Gynecol Oncol 2012;125:552-5. [Crossref] [PubMed]
- Göçmen A, Sanlikan F, Ucar MG. Robot-assisted hysterectomy vs total laparoscopic hysterectomy: a comparison of short-term surgical outcomes. Int J Med Robot 2012;8:453-7. [Crossref] [PubMed]
- Turunen H, Pakarinen P, Sjoberg J, et al. Laparoscopic vs robotic-assisted surgery for endometrial carcinoma in a centre with long laparoscopic experience. J Obstet Gynaecol 2013;33:720-4. [Crossref] [PubMed]
- Seror J, Bats AS, Huchon C, et al. Laparoscopy vs robotics in surgical management of endometrial cancer: comparison of intraoperative and postoperative complications. J Minim Invasive Gynecol 2014;21:120-5. [Crossref] [PubMed]
- Cardenas-Goicoechea J, Shepherd A, Momeni M, et al. Survival analysis of robotic versus traditional laparoscopic surgical staging for endometrial cancer. Am J Obstet Gynecol 2014;210:160.e1-e11. [Crossref] [PubMed]
- Pakish J, Soliman PT, Frumovitz M, et al. A comparison of extraperitoneal versus transperitoneal laparoscopic or robotic para-aortic lymphadenectomy for staging of endometrial carcinoma. Gynecol Oncol 2014;132:366-71. [Crossref] [PubMed]
- Colling KP, Glover JK, Statz CA, et al. Abdominal Hysterectomy: Reduced Risk of Surgical Site Infection Associated with Robotic and Laparoscopic Technique. Surg Infect (Larchmt) 2015;16:498-503. [Crossref] [PubMed]
- Frey MK, Lin JF, Stewart LE, et al. Comparison of two minimally invasive approaches to endometrial cancer staging: a single-surgeon experience. J Reprod Med 2015;60:127-34. [PubMed]
- Turner TB, Habib AS, Broadwater G, et al. Postoperative Pain Scores and Narcotic Use in Robotic-assisted Versus Laparoscopic Hysterectomy for Endometrial Cancer Staging. J Minim Invasive Gynecol 2015;22:1004-10. [Crossref] [PubMed]
- Barrie A, Freeman AH, Lyon L, et al. Classification of Postoperative Complications in Robotic-assisted Compared With Laparoscopic Hysterectomy for Endometrial Cancer. J Minim Invasive Gynecol 2016;23:1181-8. [Crossref] [PubMed]
- Mäenpää MM, Nieminen K, Tomas EI, et al. Robotic-assisted vs traditional laparoscopic surgery for endometrial cancer: a randomized controlled trial. Am J Obstet Gynecol 2016;215:588.e1-e7. [Crossref] [PubMed]
- Holtz DO, Miroshnichenko G, Finnegan MO, et al. Endometrial cancer surgery costs: robot vs laparoscopy. J Minim Invasive Gynecol 2010;17:500-3. [Crossref] [PubMed]
- Shah NT, Wright KN, Jonsdottir GM, et al. The Feasibility of Societal Cost Equivalence between Robotic Hysterectomy and Alternate Hysterectomy Methods for Endometrial Cancer. Obstet Gynecol Int 2011;2011:570464. [Crossref] [PubMed]
- Coronado PJ, Herraiz MA, Magrina JF, et al. Comparison of perioperative outcomes and cost of robotic-assisted laparoscopy, laparoscopy and laparotomy for endometrial cancer. Eur J Obstet Gynecol Reprod Biol 2012;165:289-94. [Crossref] [PubMed]
- Ind TE, Marshall C, Hacking M, et al. Introducing robotic surgery into an endometrial cancer service--a prospective evaluation of clinical and economic outcomes in a UK institution. Int J Med Robot 2016;12:137-44. [Crossref] [PubMed]
- Mendivil AA, Rettenmaier MA, Abaid LN, et al. A comparison of open surgery, robotic-assisted surgery and conventional laparoscopic surgery in the treatment of morbidly obese endometrial cancer patients. JSLS 2015;19:e2014.00001.
- Corrado G, Vizza E, Cela V, et al. Laparoscopic versus robotic hysterectomy in obese and extremely obese patients with endometrial cancer: A multi-institutional analysis. Eur J Surg Oncol 2018;44:1935-41. [Crossref] [PubMed]
- El-Achi V, Weishaupt J, Carter J, et al. Robotic versus laparoscopic hysterectomy in morbidly obese women for endometrial cancer. J Robot Surg 2020; Epub ahead of print. [Crossref] [PubMed]
- Zakhari A, Czuzoj-Shulman N, Spence AR, et al. Laparoscopic and robot-assisted hysterectomy for uterine cancer: a comparison of costs and complications. Am J Obstet Gynecol 2015;213:665.e1-7. [Crossref] [PubMed]
- Beck TL, Schiff MA, Goff BA, et al. Robotic, Laparoscopic, or Open Hysterectomy: Surgical Outcomes by Approach in Endometrial Cancer. J Minim Invasive Gynecol 2018;25:986-93. [Crossref] [PubMed]
- Johansson CYM, Chan FKH. Robotic-assisted versus conventional laparoscopic hysterectomy for endometrial cancer. Eur J Obstet Gynecol Reprod Biol X 2020;8:100116. [Crossref] [PubMed]
- Abitbol J, Cohn R, Hunter S, et al. Minimizing pain medication use and its associated costs following robotic surgery. Gynecol Oncol 2017;144:187-92. [Crossref] [PubMed]
- Leitao MM Jr, Malhotra V, Briscoe G, et al. Postoperative pain medication requirements in patients undergoing computer-assisted ("Robotic") and standard laparoscopic procedures for newly diagnosed endometrial cancer. Ann Surg Oncol 2013;20:3561-7. [Crossref] [PubMed]
- Cybulska P, Schiavone MB, Sawyer B, et al. Trocar site hernia development in patients undergoing robotically assisted or standard laparoscopic staging surgery for endometrial cancer. Gynecol Oncol 2017;147:371-4. [Crossref] [PubMed]
- Behbehani S, Suarez-Salvador E, Buras M, et al. Mortality Rates in Laparoscopic and Robotic Gynecologic Oncology Surgery: A Systemic Review and Meta-analysis. J Minim Invasive Gynecol 2019;26:1253-67.e4. [Crossref] [PubMed]
- Zullo F, Palomba S, Russo T, et al. A prospective randomized comparison between laparoscopic and laparotomic approaches in women with early stage endometrial cancer: a focus on the quality of life. Am J Obstet Gynecol 2005;193:1344-52. [Crossref] [PubMed]
- Herling SF, Moller AM, Palle C, et al. Health-related quality of life after robotic-assisted laparoscopic hysterectomy for women with endometrial cancer--A prospective cohort study. Gynecol Oncol 2016;140:107-13. [Crossref] [PubMed]
- Abitbol J, Lau S, Ramanakumar AV, et al. Prospective quality of life outcomes following robotic surgery in gynecologic oncology. Gynecol Oncol 2014;134:144-9. [Crossref] [PubMed]
- Ferguson SE, Panzarella T, Lau S, et al. Prospective cohort study comparing quality of life and sexual health outcomes between women undergoing robotic, laparoscopic and open surgery for endometrial cancer. Gynecol Oncol 2018;149:476-83. [Crossref] [PubMed]
- Graves N, Janda M, Merollini K, et al. The cost-effectiveness of total laparoscopic hysterectomy compared to total abdominal hysterectomy for the treatment of early stage endometrial cancer. BMJ Open 2013;3:e001884. [Crossref] [PubMed]
- Song J, Le T, Hopkins L, et al. A comparison of disease recurrence between robotic versus laparotomy approach in patients with intermediate-risk endometrial cancer. Int J Gynecol Cancer 2020;30:160-6. [Crossref] [PubMed]
- Koskas M, Jozwiak M, Fournier M, et al. Long-term oncological safety of minimally invasive surgery in high-risk endometrial cancer. Eur J Cancer 2016;65:185-91. [Crossref] [PubMed]
- Nasioudis D, Heyward QD, Haggerty AF, et al. Surgical and oncologic outcomes of minimally invasive surgery for stage I high-grade endometrial cancer. Surg Oncol 2020;34:7-12. [Crossref] [PubMed]
- Monterossi G, Ghezzi F, Vizza E, et al. Minimally Invasive Approach in Type II Endometrial Cancer: Is It Wise and Safe? J Minim Invasive Gynecol 2017;24:438-45. [Crossref] [PubMed]
- Papathemelis T, Oppermann H, Grafl S, et al. Long-term outcome of patients with intermediate- and high-risk endometrial cancer after pelvic and paraaortic lymph node dissection: a comparison of laparoscopic vs. open procedure. J Cancer Res Clin Oncol 2020;146:961-9. [Crossref] [PubMed]
- Jørgensen SL, Mogensen O, Wu CS, et al. Survival after a nationwide introduction of robotic surgery in women with early-stage endometrial cancer: a population-based prospective cohort study. Eur J Cancer 2019;109:1-11. [Crossref] [PubMed]
- Chambers LM, Carr C, Freeman L, et al. Does surgical platform impact recurrence and survival? A study of utilization of multiport, single-port, and robotic-assisted laparoscopy in endometrial cancer surgery. Am J Obstet Gynecol 2019;221:243.e1-e11. [Crossref] [PubMed]
- Siesto G, Romano F, Ieda NP, et al. Survival outcomes after surgical management of endometrial cancer: Analysis after the first 10-year experience of robotic surgery in a single center. Int J Med Robot 2020;16:1-9. [Crossref] [PubMed]
- Gao Q, Guo L, Wang B. The Pathogenesis and Prevention of Port-Site Metastasis in Gynecologic Oncology. Cancer Manag Res 2020;12:9655-63. [Crossref] [PubMed]
- Barraez D, Godoy H, McElrath T, et al. Low incidence of port-site metastasis after robotic assisted surgery for endometrial cancer staging: descriptive analysis. J Robot Surg 2015;9:91-5. [Crossref] [PubMed]
- Madhvani K, Curnow T, Carpenter T. Route of hysterectomy: a retrospective, cohort study in English NHS Hospitals from 2011 to 2017. BJOG 2019;126:795-802. [Crossref] [PubMed]
- Mabrouk M, Frumovitz M, Greer M, et al. Trends in laparoscopic and robotic surgery among gynecologic oncologists: A survey update. Gynecol Oncol 2009;112:501-5. [Crossref] [PubMed]
- Lau S, Vaknin Z, Ramana-Kumar AV, et al. Outcomes and cost comparisons after introducing a robotics program for endometrial cancer surgery. Obstet Gynecol 2012;119:717-24. [Crossref] [PubMed]
- Casarin J, Multinu F, Ubl DS, et al. Adoption of Minimally Invasive Surgery and Decrease in Surgical Morbidity for Endometrial Cancer Treatment in the United States. Obstet Gynecol 2018;131:304-11. [Crossref] [PubMed]
- Casarin J, Song C, Multinu F, et al. Implementing robotic surgery for uterine cancer in the United States: Better outcomes without increased costs. Gynecol Oncol 2020;156:451-8. [Crossref] [PubMed]
- Jørgensen SL, Mogensen O, Wu C, et al. Nationwide Introduction of Minimally Invasive Robotic Surgery for Early-Stage Endometrial Cancer and Its Association With Severe Complications. JAMA Surg 2019;154:530-8. [Crossref] [PubMed]
- Bouwman F, Smits A, Lopes A, et al. The impact of BMI on surgical complications and outcomes in endometrial cancer surgery--an institutional study and systematic review of the literature. Gynecol Oncol 2015;139:369-76. [Crossref] [PubMed]
- Suidan RS, He W, Sun CC, et al. Impact of body mass index and operative approach on surgical morbidity and costs in women with endometrial carcinoma and hyperplasia. Gynecol Oncol 2017;145:55-60. [Crossref] [PubMed]
- Litta P, Fabris AM, Breda E, et al. Laparoscopic surgical staging of endometrial cancer: does obesity influence feasibility and perioperative outcome? Eur J Gynaecol Oncol 2013;34:231-3. [PubMed]
- Gambacorti-Passerini ZM, Lopez-De la Manzanara Cano C, Perez Parra C, et al. Obesity in Patients with Endometrial Cancer: May It Affect the Surgical Outcomes of Laparoscopic Approach? Obes Surg 2019;29:3285-90. [Crossref] [PubMed]
- Mahdi H, Jernigan AM, Aljebori Q, et al. The impact of obesity on the 30-day morbidity and mortality after surgery for endometrial cancer. J Minim Invasive Gynecol 2015;22:94-102. [Crossref] [PubMed]
- Cunningham MJ, Dorzin E, Nguyen L, et al. Body mass index, conversion rate and complications among patients undergoing robotic surgery for endometrial carcinoma. J Robot Surg 2015;9:339-45. [Crossref] [PubMed]
- Lau S, Buzaglo K, Vaknin Z, et al. Relationship between body mass index and robotic surgery outcomes of women diagnosed with endometrial cancer. Int J Gynecol Cancer 2011;21:722-9. [Crossref] [PubMed]
- Bernardini MQ, Gien LT, Tipping H, et al. Surgical outcome of robotic surgery in morbidly obese patient with endometrial cancer compared to laparotomy. Int J Gynecol Cancer 2012;22:76-81. [Crossref] [PubMed]
- Gehrig PA, Cantrell LA, Shafer A, et al. What is the optimal minimally invasive surgical procedure for endometrial cancer staging in the obese and morbidly obese woman? Gynecol Oncol 2008;111:41-5. [Crossref] [PubMed]
- Borgfeldt C, Kalapotharakos G, Asciutto KC, et al. A population-based registry study evaluating surgery in newly diagnosed uterine cancer. Acta Obstet Gynecol Scand 2016;95:901-11. [Crossref] [PubMed]
- Fornalik H, Zore T, Fornalik N, et al. Can Teamwork and High-Volume Experience Overcome Challenges of Lymphadenectomy in Morbidly Obese Patients (Body Mass Index of 40 kg/m2 or Greater) with Endometrial Cancer?: A Cohort Study of Robotics and Laparotomy and Review of Literature. Int J Gynecol Cancer 2018;28:959-66. [Crossref] [PubMed]
- Hinshaw SJ, Gunderson S, Eastwood D, et al. Endometrial carcinoma: The perioperative and long-term outcomes of robotic surgery in the morbidly obese. J Surg Oncol 2016;114:884-7. [Crossref] [PubMed]
- Leitao MM, Narain WR, Boccamazzo D, et al. Impact of Robotic Platforms on Surgical Approach and Costs in the Management of Morbidly Obese Patients with Newly Diagnosed Uterine Cancer. Ann Surg Oncol 2016;23:2192-8. [Crossref] [PubMed]
- Nevadunsky N, Clark R, Ghosh S, et al. Comparison of robot-assisted total laparoscopic hysterectomy and total abdominal hysterectomy for treatment of endometrial cancer in obese and morbidly obese patients. J Robot Surg 2010;4:247-52. [Crossref] [PubMed]
- Seamon LG, Bryant SA, Rheaume PS, et al. Comprehensive surgical staging for endometrial cancer in obese patients: comparing robotics and laparotomy. Obstet Gynecol 2009;114:16-21. [Crossref] [PubMed]
- Subramaniam A, Kim KH, Bryant SA, et al. A cohort study evaluating robotic versus laparotomy surgical outcomes of obese women with endometrial carcinoma. Gynecol Oncol 2011;122:604-7. [Crossref] [PubMed]
- Tang KY, Gardiner SK, Gould C, et al. Robotic surgical staging for obese patients with endometrial cancer. Am J Obstet Gynecol 2012;206:513.e1-6. [Crossref] [PubMed]
- Chan JK, Gardner AB, Taylor K, et al. Robotic versus laparoscopic versus open surgery in morbidly obese endometrial cancer patients - a comparative analysis of total charges and complication rates. Gynecol Oncol 2015;139:300-5. [Crossref] [PubMed]
- Cusimano MC, Simpson AN, Dossa F, et al. Laparoscopic and robotic hysterectomy in endometrial cancer patients with obesity: a systematic review and meta-analysis of conversions and complications. Am J Obstet Gynecol 2019;221:410-28.e19. [Crossref] [PubMed]
- Galaal K, Donkers H, Bryant A, et al. Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database Syst Rev 2018;10:CD006655. [Crossref] [PubMed]
- Bishop EA, Java JJ, Moore KN, et al. Surgical outcomes among elderly women with endometrial cancer treated by laparoscopic hysterectomy: a NRG/Gynecologic Oncology Group study. Am J Obstet Gynecol 2018;218:109.e1-e11. [Crossref] [PubMed]
- Doo DW, Guntupalli SR, Corr BR, et al. Comparative Surgical Outcomes for Endometrial Cancer Patients 65 Years Old or Older Staged With Robotics or Laparotomy. Ann Surg Oncol 2015;22:3687-94. [Crossref] [PubMed]
- Lavoue V, Zeng X, Lau S, et al. Impact of robotics on the outcome of elderly patients with endometrial cancer. Gynecol Oncol 2014;133:556-62. [Crossref] [PubMed]
- Backes FJ, ElNaggar AC, Farrell MR, et al. Perioperative Outcomes for Laparotomy Compared to Robotic Surgical Staging of Endometrial Cancer in the Elderly: A Retrospective Cohort. Int J Gynecol Cancer 2016;26:1717-21. [Crossref] [PubMed]
- Lindfors A, Akesson A, Staf C, et al. Robotic vs Open Surgery for Endometrial Cancer in Elderly Patients: Surgical Outcome, Survival, and Cost Analysis. Int J Gynecol Cancer 2018;28:692-9. [Crossref] [PubMed]
- Scribner DR Jr, Walker JL, Johnson GA, et al. Surgical management of early-stage endometrial cancer in the elderly: is laparoscopy feasible? Gynecol Oncol 2001;83:563-8. [Crossref] [PubMed]
- Bijen CB, de Bock GH, Vermeulen KM, et al. Laparoscopic hysterectomy is preferred over laparotomy in early endometrial cancer patients, however not cost effective in the very obese. Eur J Cancer 2011;47:2158-65. [Crossref] [PubMed]
- Bogani G, Cromi A, Uccella S, et al. Laparoscopic staging in women older than 75 years with early-stage endometrial cancer: comparison with open surgical operation. Menopause 2014;21:945-51. [Crossref] [PubMed]
- Guy MS, Sheeder J, Behbakht K, et al. Comparative outcomes in older and younger women undergoing laparotomy or robotic surgical staging for endometrial cancer. Am J Obstet Gynecol 2016;214:350.e1-e10. [Crossref] [PubMed]
- Bourgin C, Lambaudie E, Houvenaeghel G, et al. Impact of age on surgical staging and approaches (laparotomy, laparoscopy and robotic surgery) in endometrial cancer management. Eur J Surg Oncol 2017;43:703-9. [Crossref] [PubMed]
- Lowe MP, Johnson PR, Kamelle SA, et al. A multiinstitutional experience with robotic-assisted hysterectomy with staging for endometrial cancer. Obstet Gynecol 2009;114:236-43. [Crossref] [PubMed]
- Siesto G, Uccella S, Ghezzi F, et al. Surgical and survival outcomes in older women with endometrial cancer treated by laparoscopy. Menopause 2010;17:539-44. [Crossref] [PubMed]
- Frey MK, Ihnow SB, Worley MJ Jr, et al. Minimally invasive staging of endometrial cancer is feasible and safe in elderly women. J Minim Invasive Gynecol 2011;18:200-4. [Crossref] [PubMed]
- Zeng XZ, Lavoue V, Lau S, et al. Outcome of robotic surgery for endometrial cancer as a function of patient age. Int J Gynecol Cancer 2015;25:637-44. [Crossref] [PubMed]
- Hotton J, Koual M, Gosset M, et al. Outcomes of robotic surgery for endometrial cancer in elderly women. Surg Oncol 2020;33:24-9. [Crossref] [PubMed]
- Gallotta V, Conte C, D'Indinosante M, et al. Robotic Surgery in Elderly and Very Elderly Gynecologic Cancer Patients. J Minim Invasive Gynecol 2018;25:872-7. [Crossref] [PubMed]
- Mendes V, Bruyere F, Escoffre JM, et al. Experience implication in subjective surgical ergonomics comparison between laparoscopic and robot-assisted surgeries. J Robot Surg 2020;14:115-21. [Crossref] [PubMed]
- Catanzarite T, Tan-Kim J, Menefee SA. Ergonomics in gynecologic surgery. Curr Opin Obstet Gynecol 2018;30:432-40. [Crossref] [PubMed]
- Lee MR, Lee GI. Does a robotic surgery approach offer optimal ergonomics to gynecologic surgeons?: a comprehensive ergonomics survey study in gynecologic robotic surgery. J Gynecol Oncol 2017;28:e70. [Crossref] [PubMed]
- Eltabbakh GH. Effect of surgeon's experience on the surgical outcome of laparoscopic surgery for women with endometrial cancer. Gynecol Oncol 2000;78:58-61. [Crossref] [PubMed]
- Fowler JM. The role of laparoscopic staging in the management of patients with early endometrial cancer. Gynecol Oncol 1999;73:1-3. [Crossref] [PubMed]
- Holub Z, Jabor A, Bartos P, et al. Laparoscopic surgery in women with endometrial cancer: the learning curve. Eur J Obstet Gynecol Reprod Biol 2003;107:195-200. [Crossref] [PubMed]
- Yohannes P, Rotariu P, Pinto P, et al. Comparison of robotic versus laparoscopic skills: is there a difference in the learning curve? Urology 2002;60:39-45; discussion 45. [Crossref] [PubMed]
- Seamon LG, Cohn DE, Richardson DL, et al. Robotic hysterectomy and pelvic-aortic lymphadenectomy for endometrial cancer. Obstet Gynecol 2008;112:1207-13. [Crossref] [PubMed]
- Lim PC, Kang E, Park DH. Learning curve and surgical outcome for robotic-assisted hysterectomy with lymphadenectomy: case-matched controlled comparison with laparoscopy and laparotomy for treatment of endometrial cancer. J Minim Invasive Gynecol 2010;17:739-48. [Crossref] [PubMed]
- Rocconi RP, Meredith C, Finan MA. Evaluation of the learning curve of total robotic hysterectomy with or without lymphadenectomy for a gynecologic oncology service. J Robot Surg 2011;5:189-93. [Crossref] [PubMed]
- Mäenpää M, Nieminen K, Tomas E, et al. Implementing robotic surgery to gynecologic oncology: the first 300 operations performed at a tertiary hospital. Acta Obstet Gynecol Scand 2015;94:482-8. [Crossref] [PubMed]
- Desille-Gbaguidi H, Hebert T, Paternotte-Villemagne J, et al. Overall care cost comparison between robotic and laparoscopic surgery for endometrial and cervical cancer. Eur J Obstet Gynecol Reprod Biol 2013;171:348-52. [Crossref] [PubMed]
- Korsholm M, Gyrd-Hansen D, Mogensen O, et al. Long term resource consequences of a nationwide introduction of robotic surgery for women with early stage endometrial cancer. Gynecol Oncol 2019;154:411-9. [Crossref] [PubMed]
- Wright JD, Burke WM, Wilde ET, et al. Comparative effectiveness of robotic versus laparoscopic hysterectomy for endometrial cancer. J Clin Oncol 2012;30:783-91. [Crossref] [PubMed]
- Leitao MM Jr, Bartashnik A, Wagner I, et al. Cost-effectiveness analysis of robotically assisted laparoscopy for newly diagnosed uterine cancers. Obstet Gynecol 2014;123:1031-7. [Crossref] [PubMed]
- Lundin ES, Carlsson P, Wodlin NB, et al. Cost-effectiveness of robotic hysterectomy versus abdominal hysterectomy in early endometrial cancer. Int J Gynecol Cancer 2020;30:1719-25. [Crossref] [PubMed]
- Salehi S, Avall-Lundqvist E, Legerstam B, et al. Robot-assisted laparoscopy versus laparotomy for infrarenal paraaortic lymphadenectomy in women with high-risk endometrial cancer: A randomised controlled trial. Eur J Cancer 2017;79:81-9. [Crossref] [PubMed]
- Leung A, Abitbol J, Ramana-Kumar AV, et al. Outside the operating room: How a robotics program changed resource utilization on the inpatient Ward. Gynecol Oncol 2017;145:102-7. [Crossref] [PubMed]
- Abitbol J, Munir A, How J, et al. The shifting trends towards a robotically-assisted surgical interface: Clinical and financial implications. Health Policy Technol 2020;9:157-65. [Crossref]
- Avondstondt AM, Wallenstein M, D'Adamo CR, et al. Change in cost after 5 years of experience with robotic-assisted hysterectomy for the treatment of endometrial cancer. J Robot Surg 2018;12:93-6. [Crossref] [PubMed]
- Rao PP. Robotic surgery: new robots and finally some real competition! World J Urol 2018;36:537-41. [Crossref] [PubMed]
- Samalavicius NE, Janusonis V, Siaulys R, et al. Robotic surgery using Senhance((R)) robotic platform: single center experience with first 100 cases. J Robot Surg 2020;14:371-6. [Crossref] [PubMed]
- Müller PC, Haslebacher C, Steinemann DC, et al. Image-guided minimally invasive endopancreatic surgery using a computer-assisted navigation system. Surg Endosc 2021;35:1610-7. [Crossref] [PubMed]
- Wang F, Zhang C, Guo F, et al. Navigation of Intelligent/Interactive Qualitative and Quantitative Analysis Three-Dimensional Reconstruction Technique in Laparoscopic or Robotic Assisted Partial Nephrectomy for Renal Hilar Tumors. J Endourol 2019;33:641-6. [Crossref] [PubMed]
- Andras I, Mazzone E, van Leeuwen FWB, et al. Artificial intelligence and robotics: a combination that is changing the operating room. World J Urol 2020;38:2359-66. [Crossref] [PubMed]
- Bellini V, Guzzon M, Bigliardi B, et al. Artificial Intelligence: A New Tool in Operating Room Management. Role of Machine Learning Models in Operating Room Optimization. J Med Syst 2019;44:20. [Crossref] [PubMed]
- Bhandari M, Nallabasannagari AR, Reddiboina M, et al. Predicting intra-operative and postoperative consequential events using machine-learning techniques in patients undergoing robot-assisted partial nephrectomy: a Vattikuti Collective Quality Initiative database study. BJU Int 2020;126:350-8. [Crossref] [PubMed]
- Chen IA, Ghazi A, Sridhar A, et al. Evolving robotic surgery training and improving patient safety, with the integration of novel technologies. World J Urol 2021;34:1023-30. [PubMed]
- Avgousti S, Christoforou EG, Panayides AS, et al. Medical telerobotic systems: current status and future trends. BioMedical Engineering OnLine 2016;15:96. [Crossref] [PubMed]
Cite this article as: Raban O, Bukhari YA, Gotlieb WH. Minimally invasive approach for endometrial cancer: robotic assisted vs. straight stick laparoscopy. Gynecol Pelvic Med 2022;5:8.


