
Non-gestational choriocarcinoma of the ovary complicated by dysgerminoma: a case report
Introduction
Non-gestational ovarian choriocarcinoma (NGOC) is a highly malignant tumor derived from germ cells, accounting for <0.1% of ovarian germ cell tumors. Mixed germ cell tumor (MGCT) containing a choriocarcinoma component is extremely rare. NGOC occurs most frequently in adolescents and young females: 50% of the NGOC cases occur before childhood and adolescence and can be associated with precocious puberty. It is rare in adult women and can manifest as irregular vaginal bleeding or menstrual disorders (1). Dysgerminoma is one of the most common malignant primordial germ cell tumors, accounting for 3–5% of all malignant tumors of the ovary (2). Most dysgerminoma patients are aged 10–30 years, and half of them are younger than 20 years old. The clinical manifestations of dysgerminoma are mostly related to the compression induced by the mass. The co-existence of these two tumors is even rarer. Here, we report one case of NGCO complicated by dysgerminoma, along with a literature review. We present the following article in accordance with the CARE reporting checklist (available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-59/rc).
Case presentation
The patient was an 11-year-old female student of Han ethnicity who still had not started her menstrual period. She experienced intermittent and repeated vaginal bleeding without obvious cause 6 months prior to presenting to our hospital, which was regarded as her period and was not managed clinically. Twenty days prior to presenting, she was suffering from severe pain in her right lower abdomen and began to seek medical treatment in October 2019. Computed tomography (CT) in another hospital showed that there were large, cystic or solid masses in the pelvic and abdominal cavities, which were considered to be malignant tumors originating from adnexa. Vaginal color Doppler ultrasound in the outpatient department of our hospital revealed heterogeneous weak echoes at the right adnexal area that was close to the right wall of the uterus, and these echoes reached two fingers above the umbilicus and could also be found on the right abdominal wall. Slightly rich blood flow signals were detected around and inside the masses. Notable tumor markers were the following: alpha fetoprotein (AFP), 39.3 ng/mL; carbohydrate antigen (CA)125, 54.6 U/mL; and human chorionic gonadotropin (HCG), >200,000 mIU/mL. An exploratory laparotomy was then performed. During the operation, a small amount of light-yellow ascites was seen, and the omentum and the right ovarian mass were densely adhered. After the adhesion was separated, the anteversion of the uterus was found, and there was no obvious abnormality. There was also no obvious abnormality found in her left fallopian tube or left ovary. The right fallopian tube became swollen. A huge mass sized about 16 cm in diameter was seen in the right ovary, and had a smooth surface and occupied the entire pelvic cavity. A large number of lymph nodes were enlarged in the mesentery, with the largest one having a diameter of about 1 cm; the remaining tissues had no obvious abnormalities. Part of the mass in the right ovary was removed during the operation and sent for rapid frozen section biopsy, which suggested the possibility of malignant tumors, especially germ cell tumors. Right adnexectomy was then performed, during which appendix, peritoneum on the left pelvic wall, and mesenteric lymph nodes were sent for pathology. A bleomycin + etoposide + cisplatin (BEP) regimen was applied after surgery.
The 10% formalin-fixed, paraffin-embedded (FFPE) postoperative specimens were stained with hematoxylin and eosin (HE) and observed under a light microscope. Immunohistochemistry used the EnVisionTM system (Agilent, USA), and the required antibodies, including Sal-like protein 4 (Sall-4), placental alkaline phosphatase (PLAP), CD117, D2-40, OCT3/4, vimentin, anti-endomysial antibody (EMA), HCG, AFP, glypican3, CD30, and Ki-67. The expression and location of each protein in cells were detected.
The mass in the right adnexa was 13 cm × 13 cm × 7.5 cm in size. Its surface envelope was mostly smooth and intact, and no definite ovarian tissue was seen. The cut surface was colorful: it appeared grayish-white, grayish-yellow, and grayish-red, being solid and soft in nature, with visible bleeding. Edema occurred in the 9.5 cm fallopian tube; the tube lumen was 0.3–1.2 cm in diameter, the serous membrane was smooth, and the umbrella end was open.
Extensive hemorrhage and necrosis were seen in tumor tissues, which were composed of two tumor components: one tumor component contained cytotrophoblasts and syncytiotrophoblasts, and had no placental villous tissue; the other tumor component consisted of medium-sized, round or polygonal cells. Their nuclei were medium-sized, and the cytoplasm was lightly stained or had an empty appearance similar to primordial germ cells. Tumor cells were clustered into cell nests of different sizes or arranged in strips and sheets, surrounded by different amounts of lymphocytes. These two tumor components were staggered and mixed (Figure 1).

Germ cell tumors were considered based on the histological morphological features of the HE-stained slices. Immunolabel staining of the large cells and syncytial cells revealed the following: Sall-4 (−), PLAP (+), CD117 (−), D2-40 (−), OCT3/4 (−), vimentin (−), EMA (+), HCG (+++), AFP (−), glypican3 (−), and CD30 (−); immunohistochemistry of another tumor component revealed the following: Sall-4 (+++), PLAP (+++), CD117 (+), D2-40 (++), OCT3/4 (+++), vimentin (−), EMA (−), HCG (++), AFP (−), glypican3 (−), CD30 (−), and an 80% proportion of Ki-67-positive tumor cells (Figure 2).
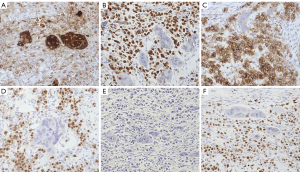
According to the microscopic morphological characteristics and the immunohistochemical staining results, the main pathological diagnosis was MGCTs containing dysgerminoma and NGCO.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
Germ cell tumors mainly occur in young women under the age of 20, with 90% being simple and 10% being mixed. MGCTs containing both dysgerminoma and NGCO are particularly rare. These can occur in prepubertal girls, with huge abdominal masses and abdominal pain as the main clinical symptoms. In our current case, the child still had her first menstrual period and presented with abdominal symptoms. Ultrasound and CT revealed huge masses in her abdomen and pelvis, which were considered to be tumors originating from adnexa. The CA125 level may increase in patients with dysgerminoma; in contrast, the blood HCG level is markedly elevated in NGCO patients, which can be associated with endocrine disorders such as precocious puberty and early pregnancy reaction-like symptoms (3).
Ovarian dysgerminoma, a rare malignancy, is a single-proliferating primitive germ cell tumor containing varying numbers of lymphocytes and phagocytes (4). Microscopically, the tumor cells are mostly strip-shaped and showed nest-like arrangement. In our case, these round or polygonal cells had clear intercell boundaries, with rich slightly stained or acidophilic cytoplasm and a large and centered nucleus. Sometimes megakaryocytes could be seen, and mitotic figures were easily visible. Immunohistochemistry of other cases has shown most dysgerminomas express OCT3/4 and PLAP, and CD117 and D2-40 can also be expressed. In our case, the microscopic morphology and immunohistochemical staining results supported the diagnosis of dysgerminoma. NGCO usually occurs unilaterally and is large in size. The cut surface of an NGCO often appears dark red in color and fragile in nature, and is often accompanied by bleeding and necrosis. Its microscopic appearance is the same as that of gestational choriocarcinoma: the tumor cells are often arranged in a sieve shape, with cytotrophoblasts and syncytiotrophoblasts being the main tumor components. The syncytiotrophoblasts are large and round; the cytoplasm is lightly stained, with or without cytoplasmic vacuoles; and the nuclei are darkly stained and may be associated with increased syncytiotrophoblastic knotting. HCG was positive. Immunohistochemically, NGCO expresses HCG, human placental lactogen (HPL), inhibin, and low-molecular-weight keratin to varying degrees, and PLAP may also be detected in a few patients. Similar microscopic morphologies and immunolabeling findings were found in our current case. Therefore, the final diagnosis was a MGCT, which contained both NGCO and dysgerminoma.
NGCO and dysgerminoma can be differentiated from other germ cell tumors as described below (1). Gestational choriocarcinoma has a characteristic biphasic trophoblastic differentiation structure, and the differentiation of simple isolated syncytiotrophoblasts can appear in multiple germ cell tumors. A key difference between gestational choriocarcinoma and NGCO is whether there is a prior history of pregnancy and whether the patient is a prepubertal girl or an adolescent. The co-existence of other tumorous germ cell components is particularly helpful for differential diagnosis in girls after menarche. DNA sequence analysis also helps to identify the common antigen of paternal human leukocytes (2). Compared to yolk sac tumor, dysgerminoma is more immature, with almost no specific differentiation, and has the characteristics of tumor cells, including homogeneity, nesting, presence of fibrous septum, and obvious lymphocyte infiltration. In contrast, the structures of yolk sac tumor cells are more diverse. The tumor cells have a moderate amount of lightly stained or hyaline cytoplasm, with prominent nucleoli and active mitosis; there are varying degrees of cell atypia including the characteristic S-D corpuscles, hyaline bodies, and papilla-shaped giant sac structure. Elevated blood AFP level and positive AFP expression also help to distinguish these two diseases (3). Embryonal carcinoma is derived from pluripotent stem cells. The tumor is composed of epithelioid cells of the blastoderm, with adenoid, tubular, papillary, or solid morphologies. The tumor cells are medium- or large-sized, with overlapping nuclei and unclear boundaries. Syncytiotrophoblast-like giant cells, located near the tumor epithelial nest or in the stroma, are easily visible. Immunohistochemistry typically reveals PLAP, OCT3/4, cytokeratin (CK), and CD30 to be positive and EMA to be negative. Mononuclear tumor cells often express AFP, and the syncytiotrophoblast-like giant cells are HCG-positive.
Dysgerminoma grows rapidly and can easily spread locally. Compared with other germ cell tumors, dysgerminoma rarely spreads to distant organs in its early stage. Therefore, early-stage dysgerminoma can be treated conservatively, and a recurrent dysgerminoma is highly sensitive to radiotherapy and chemotherapy. NGCO can easily invade adjacent organs and metastasizes through the lymphatic and blood systems. It requires postoperative adjuvant radiotherapy and chemotherapy, and the prognosis is poor. According to the recent International Federation of Gynecology and Obstetrics (FIGO) staging system, we diagnosed our patient as stage IC2 germ cell cancer (Table 1). A bleomycin + etoposide + cisplatin (BEP) regimen was applied after surgery. After five cycles of BEP during the 6-month follow-up, etoposide was changed to vincristine on the sixth cycle, and thus the BVP regimen (bleomycin + vincristine + cisplatin) was used. After three cycles of BEP regimen, serum CA125, AFP, and carcinoembryonic antigen (CEA) basically returned normal, HCG was slightly high, and the other markers showed no obvious abnormalities. After the fourth cycle of BEP, all the markers returned to normal. During chemotherapy, the child had common adverse reactions after chemotherapy, such as vomiting, hair loss, white blood cell and platelet symptoms, and no other adverse reactions. Currently, her condition is stable, and no metastasis or recurrence has occurred. After 10 months of follow-up, CA125, AFP, CEA and other indicators were normal, and the follow-up was lost. Young female patients with stage I malignant germ cell tumors tend to have a good prognosis. The 5-year survival rate can exceed 85% after standard treatments. Radiochemotherapy in patients with early-stage simple dysgerminoma can achieve a 5-year survival rate of >95%, and the prognosis of dysgerminoma with syncytiotrophoblast differentiation is similar to that of simple dysgerminoma in the same stage (5,6).
Table 1
| FIGO stage | Tumor distribution | TNM stage |
|---|---|---|
| I | Tumor confined to ovaries or fallopian tube(s) | T1 |
| IA | Tumor limited to one ovary (capsule intact) or fallopian tube. No tumor on ovarian or fallopian tube surface. No malignant cells in the ascites or peritoneal washings | T1a |
| IB | Tumor limited to both ovaries (capsules intact) or fallopian tubes. No tumor on ovarian or fallopian tube surface. No malignant cells in the ascites or peritoneal washings | T1b |
| IC | Tumor limited to one or both ovaries or fallopian tubes, with any of the following: | T1c |
| IC1 | Surgical spill intraoperatively | |
| IC2 | Capsule ruptured before surgery or tumor on ovarian or fallopian tube surface | |
| IC3 | Malignant cells present in the ascites or peritoneal washings | |
| II | Tumor involves one or both ovaries or fallopian tubes with pelvic extension (below pelvic brim) or peritoneal cancer (Tp) | T2 |
| IIA | Extension and/or implants on the uterus and/or fallopian tubes/and/or ovaries | T2a |
| IIB | Extension to other pelvic intraperitoneal tissues | T2b |
| III | Tumor involves one or both ovaries, or fallopian tubes, or primary peritoneal cancer, with cytologically or histologically confirmed spread to the peritoneum outside the pelvis and/or metastasis to the retroperitoneal lymph nodes | T3 |
| IIIA | Metastasis to the retroperitoneal lymph nodes with or without microscopic peritoneal involvement beyond the pelvis | T1, T2, T3aN1 |
| IIIA1 | Positive retroperitoneal lymph nodes only (cytologically or histologically proven) | T3a/T3aN1 |
| IIIA1(i) | Metastasis ≤10 mm in greatest dimension (note this is tumor dimension and not lymph node dimension) | T3a/T3aN1 |
| IIIA1(ii) | Metastasis N >10 mm in greatest dimension | T3b/T3bN1 |
| IIIA2 | Microscopic extrapelvic (above the pelvic brim) peritoneal involvement with or without positive retroperitoneal lymph nodes | T3c/T3cN1 |
| IIIB | Macroscopic peritoneal metastases beyond the pelvic brim N 2 cm in greatest dimension, with or without metastases to the retroperitoneal nodes | Any T, any N |
| IIIC | Macroscopic peritoneal metastases beyond the pelvic brim N 2 cm in greatest dimension, with or without metastases to the retroperitoneal nodes (Note 1) | M1 |
| Distant metastasis excluding peritoneal metastases | ||
| IVA | Pleural effusion with positive cytology | |
| IVB | Metastases to extra-abdominal organs (including inguinal lymph nodes and lymph nodes outside of abdominal cavity) (Note 2) | T3c/T3cN1 |
Note 1: this includes extension of the tumor to the capsule of the liver and spleen without parenchymal involvement of either organ; Note 2: parenchymal metastases are stage IV B. Table 1 from (FIGO) Gynecol Oncol 2014;133(3):401-4.
Few studies in China and abroad have reported on MGCTs. The clinical features of the mixed malignancies are unclear, and no consensus has been reached on the treatment options. Furthermore, the prognosis of patients cannot be systematically evaluated. Early detection and timely treatment are still the key to a good prognosis. Here, we summarize the clinicopathological features of the disease and will continue to focus on its follow-up treatment and prognosis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-59/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-59/coif). YS serves as an unpaid editorial board member of Gynecology and Pelvic Medicine from May 2019 to Apr 2021. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/ or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li LJ, Chen RL, Chi HJ. Simple nongestational choriocarcinoma of the ovary: report of one case. Chinese Journal of Practical Gynecology and Obstetrics 2005;21:444.
- Lu Z, Chen J. Introduction of WHO classification of tumours of female reproductive organs, fourth edition. Zhonghua Bing Li Xue Za Zhi 2014;43:649-50.
- Guo C, Zou LF, Wang Y, et al. Nongestational ovarian choriocarcinoma: a clinicopathological analysis of two cases. Chinese Journal of Diagnostic Pathology 2013;20:678-81.
- Li YF, Li MD, Wu QL, et al. Clinical analysis of 57 patients with ovarian dysgerminoma. Chinese Journal of Cancer 2002;21:79-82. [PubMed]
- Chen Y, Luo Y, Han C, et al. Ovarian dysgerminoma in pregnancy: A case report and literature review. Cancer Biol Ther 2018;19:649-58. [Crossref] [PubMed]
- Stafman LL, Maizlin II, Dellinger M, et al. Disparities in fertility-sparing surgery in adolescent and young women with stage I ovarian dysgerminoma. J Surg Res 2018;224:38-43. [Crossref] [PubMed]
(English Language Editor: J. Gray)
Cite this article as: Zhang C, Shen Y. Non-gestational choriocarcinoma of the ovary complicated by dysgerminoma: a case report. Gynecol Pelvic Med 2021;4:31.


