
Bilateral huge ovarian dysgerminoma with torsion: case report
Introduction
Dysgerminoma accounts for 33% of malignant ovarian germ cell tumours, and most of them occur in young women (1). Most patients with ovarian dysgerminomas often present with non-specific symptoms (2), such as pelvic mass with or without abdominal pain, but when ovarian mass is accompanied with torsion, it often leads to drastic and sudden abdominal pain (3), however, some patients may experience only mild and intermittent pains (4). Adnexal torsion of ovary is usually benign (5), it has reported that malignancy rate in ovarian torsion was about 2% (3,6). Bilateral huge ovarian dysgerminoma with torsion is extremely rare. And it is difficult to diagnose ovarian torsion through symptom or imaging examination (4). In this study, we reported a 20-year-old girl diagnosed as bilateral huge ovarian dysgerminoma accompanied with adnexal torsion, missing early surgery, presenting as repeated fever and extremely poor general condition. We present the following article in accordance with the CARE reporting checklist (available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-50/rc).
Case presentation
Informed consent was obtained from the patient for publication of this case report and accompanying images. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The timeline was shown in Table S1. The patient was a-20-year-old student (height: 170 cm, body weight: 55 kg), hadn’t had sex, with regular menstruation before. No relevant family history. More than 3 months ago, the patient touched a mass in the right lower abdomen by herself without special discomfort. One month ago, the patient felt rapid abdominal mass growth accompanied with abdominal distension, abdominal pain, loss of appetite, nausea and vomiting. Then the patient went to the local hospital. The ultrasound suggested two huge solid masses with irregular shape in the pelvic cavity. Other examination showed hemoglobin (HGB) was 80 g/L, alanine transaminase (ALT) was 626 U/L, aspartate transaminase (AST) was 673 U/L, cancer antigen 125 (CA125) was 429.1 U/L, CA199 was 53.57 U/L, beta human chorionic gonadotropin (β-hCG) was 966 mIU/mL). Diagnosis of ovarian cancer was made. Subsequently, the patient received supportive treatment and apatinib mesylate (850 mg qd). During the treatment, transaminase decreased (ALT 81 U/L, AST 22 U/L), but the masses grew extremely rapidly. The weight of the patient had reduced by more than 5 kg during the period.
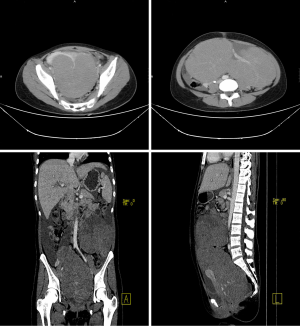
When the patient was sent to our hospital, she had recurrent fever, up to 39 °C, with small red papules scattered in the whole body, and vomiting. As Figures 1,2 showed, the ultrasound and computed tomography (CT) suggested two huge solid masses with irregular shape and abundant blood flow, and celiac effusion, pleural effusion and pericardial effusion were also detected. Meanwhile, pneumonia, leucocytes decrease, anemia (HGB 73 g/L), prolonged activated partial thromboplastin time (APTT) (40.7 s), increased d-dimer (DDI) (14.10 mg/L), increased fibrinogen degradation products (FDP) (47.40 ug/mL), decreased antithrombin III (ATIII) (45%) and elevated liver enzymes (AST 84 U/L) and decreased albumin (28.4 g/L) were detected. The diagnosis of ovarian cancer, mild anemia, liver dysfunction, pericardial effusion, bilateral pleural effusion, and right hydronephrosis was made. After a series of supportive treatment, most symptoms eased, but the general condition of the patient was still poor presenting as repeated fever, significant weight loss, cachexia, stage of exhaustion, disseminated intravascular coagulation (DIC) and impaired live function. We were always trying to find the best time for operation and preparing for it. And the patient as well as her parents were particularly eager to do the surgery.

During the surgery, we found about 800 mL of yellowish ascites, enlarged left ovary as a solid mass about 20 cm × 18 cm × 15 cm, rotating 360 degrees, with a cauliflower hyperplasia about 2 cm and a spotty area of congestion on the surface, and enlarged right ovary as a solid mass about 25 cm × 15 cm × 13 cm, rotating 360 degrees, with smooth and purple brown surface (Figure 3). The bilateral ovaries were completely destroyed by the masses, no obviously normal ovarian cortex left. Considering abnormal coagulation and extremely poor general condition of the patient, we chose to do the surgery of bilateral salpingo-oophorectomy and omentectomy without pelvic lymphadenectomy. The intraoperative bleeding was about 300 mL. No tumour remained in the naked eye. The light yellow and fish like tissue was seen in the caesarean section (Figure 4). And weight of bilateral ovaries was 2,800 g (left) and 3,000 g (right). Intraoperative frozen inspection showed low differentiated malignant tumour and malignant tumour cells in ascites (Figure 5). Postoperative pathology showed the bilateral ovarian tissues were dysgerminoma (Figure 6), and the bilateral fallopian tubes as well as the omentum were not involved. Immunohistochemistry suggested SALL4+++, OCT3/4+++, placental alkaline phosphatase (PLAP)+++, CD117+, epithelial membrane antigen (EMA)−, leukocyte common antigen (LCA)−, Ki67 80%. Diagnosis of dysgerminoma (stage IC) was confirmed. As soon as the surgery was performed, DIC, pleural effusion and ascites took a turn for the better.




Twenty days after the operation, the reexamination of chest and abdomen CT suggested the lymph nodes of abdominal aorta and left common iliac vessel were significantly increased, partly integration, considering inflammation or metastasis of lymph node, and alpha-fetoprotein (AFP) <1.3 ng/mL, carcinoembryonic antigen (CEA) <0.5 ng/mL, CA199 18.8 U/mL, total human chorionic gonadotropin (ThCG) <2.0 mIU/mL, CA125 185.6 U/mL. At that time, the patient started the first chemotherapy. We adopted the chemotherapy regimen of BEP (bleomycin 15 mg ivgtt d1/d4 + etoposide 100 mg ivgtt d1 d1–55 + cis-platinum 20 mg ivgtt d1/d3/d5, 30 mg ivgtt d2/d4), after 4 courses of this chemotherapy regimen, the reexamination of CT suggested the lymph nodes of abdominal aorta and left common iliac vessel were still increased, part integration, but obviously reduced combined with before. The patient totally received six courses of this chemotherapy regimen, after that, the tumour markers kept normal. After 5 months of the surgery, the patient had received the positron emission tomography-computed tomography (PET-CT), the results indicated that several lymph nodes were found beside the abdominal aorta and left common iliac vessels, partly integration, but no increase in glucose metabolism, no tumour metastasis or recurrence was demonstrated. As for this, the second surgery for explosion was not performed.
The patient followed up regularly in the outpatient department every 4–6 months after chemotherapy. Until the article is submitted, the patient is still alive, with normal level of serum tumour markers and physical exams, although CT also indicated increased lymph node, however, it obviously decreased compared with before.
Discussion
Although dysgerminoma accounts for only 2% of all ovarian tumours, it accounts for 33% of malignant ovarian germ cell tumours (1,2). In addition, three-fourths of dysgerminomas arise in young adults and adolescents (1,7), especially women under age 30, however, they can be found in all ages, even found during pregnancy (7,8). Most patients with ovarian dysgerminomas often present with non-specific symptoms (2), they came to hospital because of rapidly growing pelvic mass (9), but when ovarian mass is accompanied with torsion, it often leads to drastic and sudden abdominal pain (3), however, some patients may experience only mild and intermittent pains (4). In our case, the patient presented as abdominal pain and pelvic mass. Ovarian torsion is a rare gynecological emergency, usually caused by an ovarian mass twisted along the ovarian vascular pedicle (4). When ovarian torsion occurs, edema and inflammation can lead to the expansion of the ovary (6). It has been reported that adnexal torsion of ovary is usually benign (5), malignancy rate in ovarian torsion was about 2% (3,6), and most of them were in stage I (6). And bilateral huge ovarian dysgerminoma with torsion is pretty rare. So, we try to sum up some experience through this case to serve the clinical diagnosis and treatment.
The most common and convenient used preoperative imaging diagnostic method is ultrasound (2). After searching extensive literature, it indicated that the typical sonographic appearances of ovarian dysgerminoma were huge, solid, adnexal mass with lobulated and irregular echogenicity within it, accompanied with abundant blood flow signals (2). Moreover, magnetic resonance imaging (MRI) and CT may also provide auxiliary diagnosis basis. Typical appearances of CT were a low-density area with no enhancement, and the non-necrotic part with obvious enhancement (9-11). In our case, just as Figures 1,2 showed, the results were in consistent with the typical characteristics of dysgerminoma. Sometimes, ultrasound guided needle cytology may also assist the diagnosis (7). However, it is difficult to diagnose ovarian torsion through symptom or imaging examination (4). Typical CT and MRI appearances of ovarian torsion are thickening of the fallopian tubes, ascites, and the displacement of the uterus to the twisted side (4,12). When lacking the contrast of enhancement, it suggested complete torsion (13), however, it is impossible to use the agents in patients with pregnancy or severe renal insufficiency (14). Recent literature had reported that susceptibility-weighted imaging (SWI) could show hemorrhagic contents and vascular pedicle thrombosis without the presence of agents, which is helpful in diagnosing ovarian torsion. However, the diagnostic value of distinguishing malignant tumour from ovarian torsion is still limited (15).
Elevation of serum tumour markers are additional diagnostic methods of patients with ovarian dysgerminomas. A large amount of literature demonstrated that elevation of serum neuron-specific enolase (NSE), PALP, β-hCG, lactate dehydrogenase (LDH) and serum CA125 were the valuable additional diagnostic methods of patients with ovarian dysgerminomas (11,16). However, there were some literatures indicated that CA125 levels were not raised in patients with ovarian dysgerminoma (2,11,16-18). In our case, increased CA125 (429.1 U/L), CA199 (53.57 U/L) and hCG (966 mIU/mL) were detected. Recent studies reported that IL-6 levels might be a novel marker to diagnose ovarian torsion (19), it might be necessary for these who were suspected of diagnosing adnexal torsion.
The same with other tumours, diagnosis of dysgerminomas is confirmed by postoperative pathology. As showed in Figure 6, the tumour consisted of massively gathered cells with uniform and round shape, with the features that lymphocytes infiltrating connective tissue stroma. And cells had large nucleoli and prominent clear cytoplasm. Moreover, immunohistochemical staining is a very effective method to diagnose dysgerminomas, CA125, S-100 protein, cytokeratin 18, PALP, NSE and β-hCG were usually positive in dysgerminomas (17). As malignant cells usually express CD117, OCT3, and OCT4 (20), and syncytiotrophoblastic giant cells secrete LDH, they also can be considered as diagnostic characters.
Treatment of dysgerminoma is mainly based on surgery, with or without chemotherapy (21), and early surgery is quite important for patients with dysgerminoma. With laparoscopic techniques becoming more and more mature, controversy still exists (9,21). Scanning latest literatures, only one patient received laparoscopy (18). As many literatures reported, salpingo-oophorectomy with adequate staging is performed, including pelvic and abdominal exploration, peritoneal washings, lymph node sampling and biopsies of suspicion areas (9). But in our case, considering the extremely poor general condition of the patient, properly reducing the scope of surgery in order to shorten the operation time was required, so we performed the surgery of peritoneal washings, bilateral salpingo-oophorectomy and omentectomy without pelvic lymphadenectomy. Many patients with dysgerminoma are young women, preservation of fertility should be considered if it is possible (21). In our case, as for the huge tumours destroyed almost the entire ovarian tissue, and multiple torsion-induced necrosis lesions on the surface of bilateral ovaries, bilateral salpingo-oophorectomy should be performed. Recent reports indicated that although bilateral dysgerminoma accounts for 10% to 20% in dysgerminoma cases (22), if the contralateral ovary appears normal, biopsy of the contralateral ovary is not required (23). And it is reported surgical removal of the residue is required if there is a residual tumour after postoperative chemotherapy (21). For adnexal torsion, it is generally believed it would lead to obstruction of veins and lymphatic vessels, then the ovarian artery is paralyzed, and ovarian infarction resultantly. There are conservative and radical options for adnexal torsion treatment. However, it is controversial to choose detorsion or salpingo-oophorectomy, recent reports indicated that although the cortex of ovary looked similar to necrosis, most patients recovered ovulation after detorsion without thrombosis (24). But for malignant ovarian masses, it hasn’t been advised to choose detorsion, this is worth studying in the future.
Ovarian dysgerminoma is highly sensitive to chemotherapy based on platinum. 75% of patients with dysgerminoma present with stage I (25). Although all dysgerminomas are malignant, for stage 1A dysgerminomas, fertility-preserving surgery without adjuvant chemotherapy or radiotherapy was usually enough (21). The cure rate for stage 1A patients who received surgery was >90% (9). If patients of stage 1A relapsed, chemotherapy or radiation were the effective methods to cure them, although the recurrence rate for stage 1A is only 20% (26). But as International Federation of Gynecology and Obstetrics (FIGO) and some studies proposed, higher FIGO scores must receive chemotherapy (21). As the stage of our patient was IC, adjuvant chemotherapy was required. National Comprehensive Cancer Network (NCCN) suggested 3 cycles of BEP (bleomycin, etoposide, and cisplatin) for patients of stage IA who received completely resection, while 4 cycles for poor risks, and for patients of stage IB–III after resecting tumours, 3 cycles of etoposide/carboplatin can be used (21). In our case, the patient totally received 6 cycles of BEP. When dysgerminoma mixed with other malignant tumours, the determined chemotherapy hasn’t been recommended. A few reports gave their programs, three patients of mixed malignant germ cell tumour (MGCT) received chemotherapy, two of them received etoposide, ifosfamide, cisplatin (VIP), and the other one received BEP (9,10). Patients with residual lesions after cytoreductive surgery also had long-term outcome after receiving cisplatin-based chemotherapy, with overall survival of 80–90% (27). Furthermore, reported had indicated that most women whom followed up could resume normal ovarian function after received chemotherapy, while the older patients might have adverse effect on reproductive function (28). However, accurate surgical staging and early surgery are important, although surgery is not the only therapeutic method (29).
The prognosis of patients with dysgerminoma is usually good with long overall survival (9,18). Only 28% of patients had lymph node metastasis, which was a predictor of poor survival (30). Reports had indicated that the median follow-up was 127 months to 15.9 years (31). Extensive literatures had described that all recurrences of dysgerminoma occurred within 9–19 months (32), they suggested that ovarian dysgerminoma should be followed up for about 2 years, but there was another literature suggested that it may relapse after 2 years from primary diagnosis (33). NCCN suggested that physical exam (every 2–4 months) and serum tumour marker (every 2–4 months) should be followed up for dysgerminoma within two years, and when patients have been followed up for more than 2 years, only physical exam yearly should be followed up. In our case, as she received the surgery of bilateral salpingo-oophorectomy, regular detecting of sex hormone levels, bone mineral density and some other examinations are necessary. If tumour residue or recurrence was suspected, re-surgical exploration is necessary. But in our case, for the sake of PET-CT indicating no tumour metastasis or recurrence, the second surgery for explosion was not performed. Until the article is submitted, the patient is still alive without suspicious physical exams, tumour makers or obviously abnormal imaging exams.
As for our patient, preoperative examinations, such as ultrasound, CT, tumour markers, had indicated it was malignant ovarian tumour, but torsion of ovarian masses was not considered at that time. For more accurate diagnosis, IL-6 and SWI may also be detected when the diagnosis of adnexal torsion was considered. What’s more, she received chemotherapy in other hospital instead of early surgical treatment. These caused the patient to miss the timing of early surgery, which was part of the reason of her extremely poor general condition. This reminded us that diagnosis of adnexal torsion should be considered when a patient with solid adnexal mass presents with acute abdominal pain. And complete surgery should be considered at the beginning, instead of preoperational chemotherapy without determined diagnosis. However, the key point for us was to find the best time for surgery. As soon as the surgery was performed, DIC, pleural effusion and ascites took a turn for the better. It also demonstrated the necessity of early surgical treatment.
Although dysgerminomas with adnexal torsion is extremely rare, the diagnosis should be taken in consideration when a patient presents with solid adnexal mass and acute abdominal pain. ultrasound, CT, MRI and tumour markers may provide some auxiliary diagnosis basis, among them, SWI and IL-6 may play an important role. Early surgery is necessary for dysgerminomas, especially for these with huge dysgerminoma accompanied with torsion. Patients with dysgerminoma usually have long overall survival.
Acknowledgments
Funding: National Science and Technology Major Projects for Major New Drugs Innovation and Development (No. 2018ZX09733001-004). National Natural Science Foundation of China (No. 81902662)
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist (available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-50/rc).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-50/coif). XZ serves as an Editor-in-Chief of Gynecology and Pelvic Medicine. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent was obtained from the patient for publication of this case report and accompanying images. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Smith HO, Berwick M, Verschraegen CF, et al. Incidence and survival rates for female malignant germ cell tumors. Obstet Gynecol 2006;107:1075-85. [Crossref] [PubMed]
- Guerriero S, Testa AC, Timmerman D, et al. Imaging of gynecological disease (6): clinical and ultrasound characteristics of ovarian dysgerminoma. Ultrasound Obstet Gynecol 2011;37:596-602. [Crossref] [PubMed]
- Bayer AI, Wiskind AK. Adnexal torsion: can the adnexa be saved? Am J Obstet Gynecol 1994;171:1506-10. [Crossref] [PubMed]
- Chang HC, Bhatt S, Dogra VS. Pearls and pitfalls in diagnosis of ovarian torsion. Radiographics 2008;28:1355-68. [Crossref] [PubMed]
- Kokoska ER, Keller MS, Weber TR. Acute ovarian torsion in children. Am J Surg 2000;180:462-5. [Crossref] [PubMed]
- Oltmann SC, Fischer A, Barber R, et al. Pediatric ovarian malignancy presenting as ovarian torsion: incidence and relevance. J Pediatr Surg 2010;45:135-9. [Crossref] [PubMed]
- Akhtar K, Ahmad SS, Kumar A, et al. Dysgerminoma with pregnancy and viable baby: a case report. Oman Med J 2011;26:198-200. [Crossref] [PubMed]
- Nili F, Nobari N, Abdollahi A. Pseudopapillary and Macrofollicular Microscopic Growth Patterns in an Advanced Stage Ovarian Dysgerminoma: A Case Report. Iran J Pathol 2018;13:94-8. [Crossref] [PubMed]
- Al Jama FE, Al Ghamdi AA, Gasim T, et al. Ovarian tumors in children and adolescents--a clinical study of 52 patients in a university hospital. J Pediatr Adolesc Gynecol 2011;24:25-8. [Crossref] [PubMed]
- Kimura I, Togashi K, Kawakami S, et al. Ovarian torsion: CT and MR imaging appearances. Radiology 1994;190:337-41. [Crossref] [PubMed]
- Fujii S, Kaneda S, Kakite S, et al. Diffusion-weighted imaging findings of adnexal torsion: initial results. Eur J Radiol 2011;77:330-4. [Crossref] [PubMed]
- Takeuchi M, Matsuzaki K, Harada M. Susceptibility-weighted Imaging of Ovarian Torsion: A Case Report. Magn Reson Med SCI 2015;14:355-8. [Crossref] [PubMed]
- Aoki Y, Kase H, Fujita K, et al. Dysgerminoma with a slightly elevated alpha-fetoprotein level diagnosed as a mixed germ cell tumor after recurrence. Gynecol Obstet Invest 2003;55:58-60. [Crossref] [PubMed]
- Tatekawa Y, Kemmotsu H, Mouri T, et al. A case of pediatric ovarian dysgerminoma associated with high serum levels and positive immunohistochemical staining of neuron-specific enolase. J Pediatr Surg 2004;39:1437-9. [Crossref] [PubMed]
- Takeda A, Mori M, Sakai K, et al. Laparoscopic management of ovarian dysgerminoma presenting with acute abdomen caused by adnexal torsion in a 17-year-old girl. J Pediatr Adolesc Gynecol 2009;22:e9-13. [Crossref] [PubMed]
- Daponte A, Pournaras S, Hadjichristodoulou C, et al. Novel serum inflammatory markers in patients with adnexal mass who had surgery for ovarian torsion. Fertil Steril 2006;85:1469-72. [Crossref] [PubMed]
- Khandwala K, Shahid J, Nadeem N, et al. Torsion of Ovarian Dysgerminoma in a Child: Role of Computed Tomography. Cureus 2018;10:e2522. [Crossref] [PubMed]
- Chiou SY, Lev-Toaff AS, Masuda E, et al. Adnexal torsion: new clinical and imaging observations by sonography, computed tomography, and magnetic resonance imaging. J Ultrasound Med 2007;26:1289-301. [Crossref] [PubMed]
- Hoei-Hansen CE, Kraggerud SM, Abeler VM, et al. Ovarian dysgerminomas are characterised by frequent KIT mutations and abundant expression of pluripotency markers. Mol Cancer 2007;6:12. [Crossref] [PubMed]
- Deborah K, Steven C, Ronald D, et al. Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer. Version 1. 2018. NCCN Clinical Practice Guidelines in Oncology. 2018. Available online: https://www.nccn.org/patients/
- Varras M, Tsikini A, Polyzos D, et al. Internal hemorrhage caused by a twisted malignant ovarian dysgerminoma: ultrasonographic findings of a rare case and review of the literature. Clin Exp Obstet Gynecol 2004;31:73-8. [PubMed]
- Ammor A, Kisra M, Oulahyane R, et al. Ovarian tumours in children: a review of 18 cases. Afr J Paediatr Surg 2012;9:231-6. [Crossref] [PubMed]
- Lu KH, Gershenson DM. Update on the management of ovarian germ cell tumors. J Reprod Med 2005;50:417-25. [PubMed]
- Spinelli C, Pucci V, Strambi S, et al. Treatment of ovarian lesions in children and adolescents: a retrospective study of 130 cases. Pediatr Hematol Oncol 2015;32:199-206. [Crossref] [PubMed]
- Pectasides D, Pectasides E, Kassanos D. Germ cell tumors of the ovary. Cancer Treat Rev 2008;34:427-41. [Crossref] [PubMed]
- Patterson DM, Murugaesu N, Holden L, et al. A review of the close surveillance policy for stage I female germ cell tumors of the ovary and other sites. Int J Gynecol Cancer 2008;18:43-50. [Crossref] [PubMed]
- Husaini HAL, Soudy H, El Din Darwish A, et al. Pure dysgerminoma of the ovary: a single institutional experience of 65 patients. Med Oncol 2012;29:2944-8. [Crossref] [PubMed]
- Tangir J, Zelterman D, Ma W, et al. Reproductive function after conservative surgery and chemotherapy for malignant germ cell tumors of the ovary. Obstet Gynecol 2003;101:251-7. [Crossref] [PubMed]
- Mangili G, Sigismondi C, Lorusso D, et al. Is surgical restaging indicated in apparent stage IA pure ovarian dysgerminoma? The MITO group retrospective experience. Gynecol Oncol 2011;121:280-4. [Crossref] [PubMed]
- Kumar S, Shah JP, Bryant CS, et al. The prevalence and prognostic impact of lymph node metastasis in malignant germ cell tumors of the ovary. Gynecol Oncol 2008;110:125-32. [Crossref] [PubMed]
- Buskirk SJ, Schray MF, Podratz KC, et al. Ovarian dysgerminoma: a retrospective analysis of results of treatment, sites of treatment failure, and radiosensitivity. Mayo Clin Proc 1987;62:1149-57. [Crossref] [PubMed]
- Benedet JL, Bender H, Jones H, et al. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet 2000;70:209-62. [Crossref] [PubMed]
- Jeyakumar A, Cabeza R, Hindenburg A. Late recurrence in ovarian dysgerminoma with successful response to standard adjuvant chemotherapy: a case report and review of the literature. Gynecol Oncol 2001;81:314-7. [Crossref] [PubMed]
Cite this article as: Zheng H, Meng J, He Y, Tan X, Zhao X. Bilateral huge ovarian dysgerminoma with torsion: case report. Gynecol Pelvic Med 2021;4:22.


