
Surgery for recurrent ovarian cancer: expert point of view
Ovarian cancer affects 300,000 thousand women worldwide each year. Despite aggressive treatment regimens, it is still the cause of 190,000 deaths globally, which is mainly due to diagnosis at an advanced stage in the majority of cases.
The initial treatment consists of a combined approach of surgery, aiming for complete cytoreduction which is defined as removal of all macroscopic peritoneal disease, and adjuvant platinum-based chemotherapy with paclitaxel. Maintenance therapy, with bevacizumab or PARP inhibitor either alone or as combination, depends on histological type, initial extent of the disease, postoperative residual disease, the presence of BRCA mutation, or homologous recombination deficiency (HRD).
It is becoming increasingly evident that all patients with a resectable tumor burden benefit the most from initial cytoreduction with complete macroscopic resection and patients with a primarily non-resectable disease should undergo interval debulking following neoadjuvant chemotherapy (NAC). The results of the TRUST trial, which looked into that matter, will further establish the management of a large amount of newly diagnosed patients.
As of today, however, despite conducting the best possible treatment strategy, more than 75% of patients with advanced stage, relapse within 2 years after end of therapy.
Until recently, only medical treatments have been available for the recurrent setting including chemotherapy, bevacizumab or PARP inhibitors. Surgical treatment has been a marginal issue, but recent clinical trials have highlighted its importance in a selected patient cohort, therefore stating surgery as a valid option.
The concept of secondary cytoreductive surgery was introduced approximately 30 years ago by Berek et al. (1). The literature regarding this topic, including the latest one, mainly consists of retrospective studies, literature reviews and meta-analyzes with the exception of few prospective series (2,3). The scope of these studies is limited as they included patients who underwent emergency surgery for bowel obstruction, second-look procedures, incomplete surgery after chemotherapy, had progressed during chemotherapy, those with a significant residual disease as well as those recurring <6 months after the end of initial treatment. Moreover, the selection criteria of some of those retrospective studies have been quite vague.
However, results, showed better survival data for patients who have had a complete secondary cytoreduction indicating that this is the main prognostic factor together with progression free interval (PFI) (2-6). Other factors which have been shown to influence the prognosis were use of NAC (which is associated with a worse prognosis) (2), overall number of cycles of adjuvant chemotherapy (3), platinum-based chemotherapy (4), poor general health status (2,3), absence of ascites (4) and year of publication (5).
Identified parameters significantly associated with the feasibility of complete resection were patient’s general health condition (2,4), size of recurrence, extent and number of recurrent lesions, absence of ascites in the recurrent setting (2-4), tumor stage at initial diagnosis (4), complete primary cytoreduction (3) and need of bowel resection (3). These procedures usually take about 4 hours and are associated with acceptable morbidity and minimal mortality (2,3).
The rate of successful resection varied greatly from one trial to another. The studies differed in terms of level of expertise of the respective surgical teams as well as in patient selection with the rate of non-operated patients rarely stated.
The observed benefit in overall survival (OS) for patients who had undergone complete secondary resection raised the question about the role of surgery in the setting of platinum-sensitive recurrences. However, the strength of the available results was limited due to the retrospective design of the studies. The observed result could be either due to the performed intervention, the selected cohort of patients or the qualifications of the team.
The selection of patients therefore appears to be necessary. The ‘AGO score’ has been designed and validated in context of the DESKTOP studies. Factors evaluated were good general condition, complete initial surgery (or early stage) and absence of more than 500 mL ascites during recurrence (7,8). When all criteria are met, the probability of receiving a complete cytoreduction is higher than 75%. This score eliminates approximately 50% of patients with platinum-sensitive recurrence although it needs to be underlined, that a complete resection is feasible in about 43% of rejected patients.
The score designed by Tian focuses on other parameters, such as: FIGO stage, platinum-free interval and CA 125 level. Rate of complete resection is 53.4% in the low-risk group, which accounts for up to 60% of patients (9).
However, it is unclear whether survival benefit is related to surgery or to the selection of a subpopulation, which is generally associated with a better prognosis. Further investigations in a phase III trial were warranted.
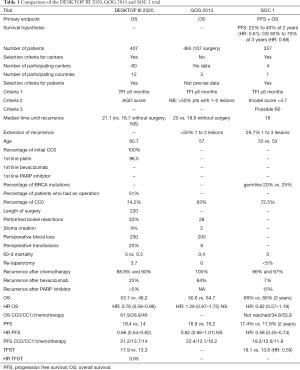
The results of three trials (GOG 213, DESKTOP III and SOC 1) are now available (Table 1), but only the GOG 213 trial has yet been published in a peer review journal (10-12). All of the above-mentioned studies have a comparable design. Patients with first platinum-sensitive recurrence were randomized to either surgery followed by platinum-based chemotherapy or chemotherapy alone. The aim of surgery was to achieve complete cytoreduction. Primary endpoint was defined as OS in the GOG 213 and DESKTOP III trials and a co-primary with OS as well as progression free survival (PFS) in the SOC 1 trial.
Full table
Patient populations were comparable in terms of age, histological types, PFI (median PFI was >20 months in two of the three studies, showing the selection of a highly platinum-sensitive population), previous treatments, etc. Notable, the ratio of patients with BRCA mutation or a HRD profile was only stated in the SOC 1 trial with a range of 20–25%.
The type of surgery performed was also comparable among the three studies in relation to the rate of digestive resections, stoma creation, perioperative blood loss and post-operative morbidity and mortality. These operations lasted 4 hours in median and a bowel resection was necessary in roughly one third of patients. They were well tolerated with a low rate of postoperative complications, readmissions, re-laparotomy and death. In GOG 213, a significant decline in quality of life and patient-reported outcomes was reported immediately after the procedure. By 6 weeks they reached parity with patients who did not undergo surgery, and they maintained parity at subsequent assessments (10).
However, these trials were distinct in terms of patient cohort and center selection. In the DESKTOP III trial, patients who qualified as operable were selected according to the ‘AGO score’, whereas the Chinese study included them using the ‘imodel score’. It is known that by using the ‘AGO score’, approximately 50% of patients with a platinum-sensitive relapse do not qualify as operable, whereas the ‘imodel’ score seems to be less restrictive. In the GOG 213 trial, patients were included according to surgeon’s opinion on operability (complete resection seemed attainable) and the percentage of excluded patients was not reported. Keeping these distinct selection criteria in mind, it is likely that the severity of recurrence differs between these trials. The SOC 1 trial reported the most severe disease population followed by the GOG 213 trial and the DESKTOP III trial with the most favorable outcome.
Regarding center selection, the included centers were specified only for the DESKTOP III and the Chinese study (where they selected the centers according to their volume). Selection of centers was not detailed in the GOG 213 trial.
Except that, different systemic therapies were applied in the respective studies. While in the DESKTOP III and SOC 1 trial mainly platinum-based chemotherapy was administered, 80% of patients in the U.S. trial received chemotherapy in combination with bevacizumab as maintenance therapy, which can be seen as another objective of the GOG 213 trial.
In terms of surgical outcome, the rate of complete resections was higher in the German and Chinese (74% and 72% respectively) than in the American trial (63%) (χ2 test, P<0.05).
Regarding PFS all trials reported a benefit for the surgery arm with remarkably similar medians of approximately 17 to 18 months. When compared to the overall study population of DESKTOP III and SOC I, the difference was significant (12 to 14 months in the standard arms). The GOG 213 trial reported a trend in favor of the surgical arm, however, without significance. This might be due to the very good results in the medical arm with a median PFS of 16 months. Finally, it should be pointed out that the highest benefit in terms of PFS was observed in patients who underwent a complete cytoreduction, with a median of 21.2 months in DESKTOP III, 22.4 months in GOG 213 and 19.2 months in SOC 1. In the GOG 213 trial, a significant difference in PFS was seen when comparing the complete surgery group to the entire no-surgery arm (HR: 0.62, 0.48–0.80).
In terms of OS, the surgical arm showed comparable results of >50 months in all three trials. Compared to the standard chemotherapy arm, a significant difference was reported in the DESKTOP III (HR: 0.76) as well as in the Chinese trial, whereas GOG 213 showed better results in OS for the chemotherapy ± bevacizumab arm (64.7 months, HR: 1.29) without significant difference. However, results for the surgery arm were comparable and consistent with the other two trials but because of their exceptional OS rate with a median of 64.7 months for the chemotherapy + bevacizumab arm, no significant difference in OS between the two arms could be reported.
Just to provide an idea, other recently published trials like SOLO 2 (13), which included only BRCA mutated patients and in the ENGOT trial recently reported by Pfisterer et al. (4), in which both arms received platinum-based chemotherapy and bevacizumab, reported a median OS of 51.7 months and 30 months, respectively. PFS in the ENGOT trial was 13 months. Although we cannot compare these studies directly, the deviation in OS for the medical arm in GOG 213 is remarkable. As well as for PFS, best results were reported in patients with complete resection. On the other hand, patients with residual tumor after surgery had comparable survival data to those who did not undergo surgery at all.
The subgroup analyses showed that only interventions leading to complete resection provided a benefit. Patients with residual disease had the same or even lower survival rates than the standard arm. This result was reported in all three trials. No other subgroup of patients has been identified that would have an advantage or disadvantage in performing an operation. However, the GOG 213 trial noted that patients suffering from serous cancer and those with a long PFI of >12 months had better survival rates in the absence of surgery (10).
Several explanations may help to understand the divergent results of DESKTOP III, SOC 1 and GOG 213.
The rate of complete resection in the U.S. trial, which is a major prognostic factor, was lower than in the other studies probably due to the absence of predefined selection criteria. It is of note that trials where the inclusion criteria for patients and centers were defined showed a benefit in the surgery arm.
A greater number of patients in the GOG 213 trial (33%) than in the DESKTOP III (26%) or the SOC 1 trial (28%) had macroscopic disease after surgery in the surgery arm. The GOG 213 study is the only trial where a majority of patients received bevacizumab in addition to chemotherapy. It raises the question of how much bevacizumab could have impacted on survival in patients who did not achieve a complete surgical remission with as consequence a non-visibility of secondary surgery benefit. Indeed, although median PFS for patients treated with surgery was in the same range in the three trials, those included in the chemotherapy arm in the GOG 213 have a superior median PFS compared to that of the two other trials, explaining the absence of difference between chemotherapy alone versus surgery + chemotherapy in the GOG 213 study. This superior outcome of patients treated by chemotherapy alone may be due to the specific addition of bevacizumab in the GOG 213 trial.
On a purely methodological level, the results of the GOG 213 trial came from an intermediate analysis with 125 events vs. 250 which have been calculated initially as necessary for the hypothesis. The confidence interval was large and the differences between “surgery” and “chemotherapy” arms were not significant for both PFS and OS. There was also an inconsistency between PFS and OS in the American trial. While the reported PFS was similar to those in the other two trials, this did not reflect in a favorable OS. Cross over between the two arms is a credible explanation.
The diagnostic tool used for the diagnosis of the second recurrence in the DESKTOP III trial might have favored the surgical arm in terms of PFS calculation. As it is known, the CT scan, which has been used for diagnosis, detects a recurrence in the setting of residual disease more easily if the patient underwent chemotherapy rather than complete surgical resection.
All three trials included patients regardless of histological subtypes. High-grade serous cancers were the majority, but 15% to 19% of the patients had low chemo sensitive types meaning low grade serous and clear cell cancers. What effect could these patients have had on the overall results? One could imagine that complete surgery is more effective than medical treatment in this subgroup.
Finally, the HRD and BRCA status of these patients should be known and the results interpreted according to the patient profile.
For PARPi naive patients, it would be interesting to compare the benefit of surgery to medical treatment with chemotherapy alone followed by maintenance therapy with PARPi ± bevacizumab. A retrospective study performed by Marchetti et al. (14) showed an improvement in survival in patients undergoing complete resection followed by chemotherapy and PARPi maintenance. Conversely, mutated patients who did not receive adjuvant PARPi (14,15) did not profit from surgery. Further questions arise like: What is the role of surgery in patients previously treated with PARPi? To answer this and the question of re-challenge, further studies have to be conducted. For now, the basis of decisions is not given due to missing evidence.
Until today, information on possible cross-over during treatment of first recurrence is missing. How many patients of the chemotherapy arm have been operated during the treatment of the recurrence? We also have very little information on the treatments received during subsequent recurrences. The results of the GOG 213 trial showed the important effect of bevacizumab at first relapse. Nevertheless, the question remains in what proportion was this drug administered for subsequent relapses and was it balanced between the arms of the study?
The application of PARP inhibitors in highly platinum-sensitive patients among the different trials should be considered as well. In SOC 1 20–25% of patients had a BRCA mutation and 10–15% of patients received a PARP inhibitor. It needs to be verified, that the use of maintenance therapies has been balanced across the different arms of these trials. The same applies for surgery which appeared to have been performed in about one-third of the patients in the SOC 1 trial for treatment of subsequent recurrences.
The selection of patients with limited lesions and carcinosis raises questions about modalities of follow-up after initial treatment. The tests and examination interval partly determined the time of diagnosis of recurrence and therefore the extent of the lesions. According to the publication of Rustin et al. (16), it is suggested to wait for clinical signs of recurrence before starting treatment. But today the situation has changed. If surgery is considered, it should be performed as early as possible to avoid wide spread lesions. Therefore, systematical clinical monitoring in predefined intervals with the help of imaging and specific tumor markers needs to be done, in particular for patients who underwent a complete surgical resection at first line and could benefit of secondary cytoreduction.
Nevertheless, we have to keep in mind the non-negligible percentage of patients who have not been selected for surgery according to the AGO or imodel score, who might reach a complete resection. This rate was 63% for the AGO score, in the absence of associated carcinosis. Even though patients might not be suitable for cytoreduction according to the score, you should discuss on operability in a highly trained team, taking into account the extent of recurrence and the procedures that have been already carried out during the initial operation.
When summarizing all the data and the results of the German and Chinese trials, they both found an improved PFS and OS in selected patients according to a specific score who have received second cytoreductive surgery which was not observed in the American trial were the patient selection process was less strict. Notably, it was confirmed by all three trials that only those patients who had a complete resection did benefit from surgery.
The take-home message is:
- Only a complete resection is of benefit to the patient. It is therefore imperative to select the patients who will be offered surgery. Patient selection could be facilitated by the application of a score like the AGO score, but should not be made by using a score only. The topography of the recurrence (meaning has a resection taken place at the spot of recurrence in the first surgery or is it an “untouched” area) and the procedures performed during first surgery including digestive resections, stoma creation and other extensive surgical procedures must also be taken into account.
- Only patients suitable for a primary surgery at relapse are considered in these trials and these comments.
- These patients should be discussed in interdisciplinary tumor boards and surgery should be performed by teams of specialist in oncologic centers. As these operations often occur after a major primary cytoreduction and frequently during treatment, they tend to be complex and often involve poly-visceral surgery. The two optimistic trials were carried out in expert teams. The positive effect and need of collaborative and multidisciplinary management of these patients has been reported several times (17).
- Risk of postoperative complication is not an issue, in case of properly selected patients in trained teams.
- If there is an indication for anti-angiogenic treatment in the event of a relapse in a patient who did not receive it initially, surgery is debatable. On the other hand, complete surgery is important in patients who have already received anti-angiogenic treatment before.
- The role of maintenance therapy with targeted, anti-angiogenic therapy or PARP inhibitor alone or in combination should be discussed given the results of recently published trials. This leads to the question if surgery would be justified in a patient not eligible for therapy with these drugs?
- If a complete resection seems difficult to achieve in an expert center, chemotherapy followed by maintenance treatment should be preferred.
For the future, a meta-analysis of these three trials will be interesting. Furthermore, the results of an ongoing Japanese trial and the Dutch SOCceR trial are of special interest. It will be another challenge for the future to determine the role of surgery in selected cases of second or third recurrence.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Federico Ferrari) for the series “Surgical Approaches for Gynecologic Cancers” published in Gynecology and Pelvic Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm-2020-13/coif). The series “Surgical Approaches for Gynecologic Cancers” was commissioned by the editorial office without any funding or sponsorship. FL serves as an unpaid editorial board member of Gynecology and Pelvic Medicine from Sep 2019 to Aug 2021. JF reports personal fees from GSK, Astra Zeneca, Sanofi, MSD, BMS, Pfizer, Ipsen, Astellas, Roche, Clovis, outside the submitted work. EPL reports personal fees and non-financial support from Astra-zeneca, personal fees and non-financial support from Tesaro, personal fees from Clovis, personal fees and non-financial support from Roche, personal fees from Incyte, personal fees from Pfizer, other from ARCAGY-Research, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Berek JS, Hacker NF, Lagasse LD, et al. Survival of patients following secondary cytoreductive surgery in ovarian cancer. Obstet Gynecol 1983;61:189-93. [PubMed]
- Eisenkop SM, Friedman RL, Wang HJ. Secondary cytoreductive surgery for recurrent ovarian cancer. A prospective study. Cancer 1995;76:1606-14. [Crossref] [PubMed]
- Zang RY, Li ZT, Tang J, et al. Secondary cytoreductive surgery for patients with relapsed epithelial ovarian carcinoma: who benefits? Cancer 2004;100:1152-61. [Crossref] [PubMed]
- Pfisterer J, Shannon CM, Baumann K, et al. Bevacizumab and platinum-based combinations for recurrent ovarian cancer: a randomised, open-label, phase 3 trial. Lancet Oncol 2020;21:699-709. [Crossref] [PubMed]
- Bristow RE, Puri I, Chi DS. Cytoreductive surgery for recurrent ovarian cancer: a meta-analysis. Gynecol Oncol 2009;112:265-74. [Crossref] [PubMed]
- Lorusso D, Mancini M, Di Rocco R, et al. The role of secondary surgery in recurrent ovarian cancer. Int J Surg Oncol 2012;2012:613980 [Crossref] [PubMed]
- Harter P, du Bois A, Hahmann M, et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol 2006;13:1702-10. [Crossref] [PubMed]
- Harter P, Sehouli J, Reuss A, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer 2011;21:289-95. [Crossref] [PubMed]
- Tian WJ, Chi DS, Sehouli J, et al. A risk model for secondary cytoreductive surgery in recurrent ovarian cancer: an evidence-based proposal for patient selection. Ann Surg Oncol 2012;19:597-604. [Crossref] [PubMed]
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med 2019;381:1929-39. [Crossref] [PubMed]
- Du Bois A, Vergote I, Ferron G, et al. Randomized controlled phase III study evaluating the impact of secondary cytoreductive surgery in recurrent ovarian cancer: AGO DESKTOP III/ENGOT ov20. J Clin Oncol 2017;35:5501. [Crossref]
- Zang R, Zhu J, Shi T, et al. A randomized phase III trial of secondary cytoreductive surgery in later recurrent ovarian cancer: SOC1/SGOG-OV2. J Clin Oncol 2020;38:6001. [Crossref]
- Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1274-84. [Crossref] [PubMed]
- Marchetti C, De Leo R, Musella A, et al. BRCA mutation status to personalize management of recurrent ovarian cancer: a multicenter study. Ann Surg Oncol 2018;25:3701-8. [Crossref] [PubMed]
- Estati FL, Pirolli R, de Alencar VTL, et al. Impact of BRCA1/2 mutations on the efficacy of secondary cytoreductive surgery. Ann Surg Oncol 2020; Epub ahead of print. [Crossref] [PubMed]
- Rustin G, van der Burg M, Griffin C, et al. Early versus delayed treatment of relapsed ovarian cancer. Lancet 2011;377:380-1. [Crossref] [PubMed]
- Burton E, Chase D, Yamamoto M, et al. Surgical management of recurrent ovarian cancer: the advantage of collaborative surgical management and a multidisciplinary approach. Gynecol Oncol 2011;120:29-32. [Crossref] [PubMed]
Cite this article as: Schütz A, Taumberger N, Pautier P, Florence J, Ferron G, Classe JM, Pujade-Lauraine E, Asselain B, Lecuru F. Surgery for recurrent ovarian cancer: expert point of view. Gynecol Pelvic Med 2021;4:18.


