
Radiotherapy in uterine sarcoma: a narrative review of international guidelines
Introduction
Uterine sarcomas (US) are rare and heterogeneous malignant mesenchymal tumors. They account for 3% to 7% of uterine cancers. The annual incidence is almost 2 cases/100,000 inhabitants and the age at diagnosis ranges between 50 and 70 years (1,2).
Main histotypes include: leiomyosarcoma (LM), low-grade endometrial stromal sarcoma (LG-ESS), high-grade endometrial stromal sarcoma (HG-ESS) and undifferentiated endometrial sarcoma (UES) according to last WHO classification (3). Carcinosarcomas (CS) were categorized as US until 2000s but now they are included in high-grade epithelial tumors, being that they contain a malignant epithelial component (4). Nevertheless, we included them in this review because they are still included in many retrospective studies on US and in some guidelines, together with the other subtypes.
The diagnosis of US is often made after surgery, even if ultrasound and magnetic resonance imaging (MRI) could give the suspicion of mesenchymal malignant tumor, above all in patients with rapidly growing uterine mass. Pathological examination should be performed in centers with established expertise in this field.
LM is the most common US (60%), arising from myometrial muscle, occurring at the median age of 50 years (1,5). LMs are aggressive tumors with dismal prognosis, depending above all on stage (2). LG-ESS represents almost 10% of US and arises from endometrial stroma. It is a slow-growing indolent disease, that usually affects 45–58 years old women (1,6). A prolonged follow up is mandatory because of the risk of long-term recurrences, also after decades. HG-ESS and UES, always originating from endometrial stroma, account for 5% of all US. They have an aggressive behavior and poor prognosis, with a median age at diagnosis among 55 and 60 years (1,7). Most patients present with advanced disease at diagnosis, with 1–2 years median overall survival (OS) (2,7). Lastly, CS are high grade aggressive tumors, typically arising in postmenopausal women, with both malignant epithelial and mesenchymal components. Two third of patients are diagnosed with advanced stage disease (8).
Standard local treatment of US and CS, in patients without metastases, is total hysterectomy with or without bilateral salpingo-oophorectomy (9,10) avoiding laparoscopic morcellation due to the higher risk of recurrence and metastasis (11,12). Systematic lymphadenectomy has not been demonstrated to be useful and therefore is not routinely indicated (1,9,10), except for CS where the risk of nodal involvement is relatively high and an impact on survival has been hypothesized (8). Locally advanced or metastatic disease are usually treated with systemic therapies, such as chemotherapy (CHT) or hormonal therapy (HT) according to the histological type. In this setting, local therapies as surgery or radiotherapy (RT) may have a palliative role.
Neoadjuvant CHT has no evidence to date, and it is only considered in some guidelines with the aim of cytoreduction in locally advanced but potentially resectable disease (1,13).
Adjuvant CHT is always recommended in CS, also in early stages, while in other US it is proposed only in advanced stages or in patients with high-risk features (large tumors or deep myometrial invasion mainly) (1,4,14,15). The more effective drugs are doxorubicin, gemcitabine, docetaxel, ifosfamide, and paclitaxel.
In LG-ESS, and in other US with hormonal receptors expression, HT is suggested after surgery, mainly based on progestogens but also aromatase inhibitors (1,4,13).
In patients with high risk of local relapse (tumor rupture, large tumors or with involvement of cervix, parametria, uterine serosa) RT is suggested after surgery above all in CS, LM, HG-ESS, and UES (4,5,10).
US are a rare heterogeneous disease and their management it is itself heterogeneous, probably due to the lack of strong evidence. In this review we collected all available international clinical practice guidelines and consensus conference about US and CS from PubMed database and other oncological societies, in order to focus on the RT role in all settings. We present the following article in accordance with the Narrative Review reporting checklist (available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-65/rc).
Material and methods
We searched for available clinical guidelines about US treatments, without time restrictions, in PubMed database and from other oncological societies. The search strategy in PubMed was: ((uterine sarcoma) OR (uterine carcinosarcoma)) AND (guideline). We decided to also cover guidelines about CS, because some authors included them in US guidelines and many retrospective studies included patients with CS among US. We included international guidelines only if an English version was available.
Two authors independently examined full text of all articles potentially useful in this analysis. In case of disagreements in this selection, a final decision was taken through a discussion with a third author.
Analyzing the included papers, we collected, for each of them, indications for primary, adjuvant and neoadjuvant treatment, divided by histological subtype. If available, we also reported the design of the studies on which the guidelines were based.
Discussion
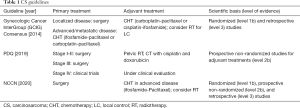
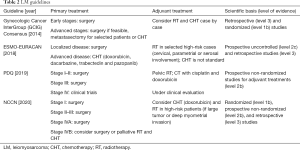
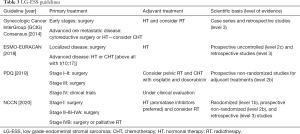
We performed a review of international guidelines on US treatment, mainly focusing on RT. In total, seven international guidelines were identified (4-9,16). The Gynecologic Cancer InterGroup (GCIG) published four different papers for each different histological US type (6-8,16). CS treatment was described in three international guidelines (4,5,8), and LM and LG-ESS were included in all of them (4-6,9,16), HG-ESS and UES were included in three guidelines (4,7,9). Tables 1-4 summarize the indications of guidelines for CS, LM, LG-ESS and HG-ESS/UES, respectively.
Full table
Full table
Full table

Full table
CS treatment
Three guidelines described CS treatment (4,5,8) with different indications for early and advanced stages only in two of them (5,8). The primary treatment is usually total hysterectomy with bilateral salpingo-oophorectomy, pelvic and para aortic nodal dissection, and omentectomy.
There is not a clear consensus on adjuvant therapies but considering the high rate of local recurrences and of distant metastases, all guidelines suggest adjuvant CHT, also in early stages due to the benefit on progression free survival (PFS) and OS, as reported in one phase III trial (17) and one Cochrane metanalysis (18).
RT
RT has to be considered in high-risk local relapse patients (myometrial invasion, advanced stage, lymphadenectomy not performed), possibly with concomitant or sequential CHT (8).
Palliative RT can improve quality of life in advanced/inoperable disease, and in recurrent or metastatic patients.
LM treatment
All the included guidelines (4,5,9,16) considered LM therapy. Surgery as total hysterectomy with bilateral resection of the adnexa is always the first treatment when feasible based on tumor stage.
Conflicting opinions exist about adjuvant treatments. One guideline suggests CHT in all patients after surgery (5), others only in selected high-risk patients with advanced stage, micro or macroscopic residual after surgery, morcellation, and high mitotic index (4). Some guidelines did not suggest CHT as standard adjuvant approach (9,16).
RT
Adjuvant RT increases LC but there is no benefit on OS, therefore some guidelines suggested to consider it in high-risk patients with tumor rupture during surgery, residual disease after surgery, higher stages, and cervical/parametrial/serosal involvement (4,9,16).
Palliative RT has to be considered in patients with local relapses or metastases.
LG-ESS treatment
All guidelines (4-6,9) included LG-ESS treatment. The first approach in localized disease remains surgery based on total hysterectomy with bilateral salpingo-oophorectomy. Systematic lymphadenectomy did not demonstrate a benefit in retrospective studies and so it is not routinely recommended, unless enlarged nodes are evident at surgical exploration (6).
For the high rate of hormone receptor positivity in this specific histological subtype, adjuvant treatment is usually based on HT with progestogens, aromatase inhibitors, megestrol acetate, or gonadotropin releasing hormone analogues (GnRH). Adjuvant HT is recommended in all patients in some guidelines (6,9), and from stage II in NCCN guideline (4).
Among LG-ESS there is a subtype with a particular genetic alteration, that is t(10;17), with a more aggressive behavior and an increased risk of metastases. It is usually HT resistant and therefore it is suggested to consider CHT for these patients, particularly in the metastatic setting (9).
RT
In LG-ESS, the incidence of distant metastases is higher compared to the rate of local recurrences, and therefore RT has a minor role in this setting, though some retrospective studies suggested improved LC after RT (6,9). Moreover, the paucity of published data suggest that RT is not offered as a standard adjuvant therapy.
Some guidelines suggest RT in high risk for local relapse patients, mainly those with stage III–IV or with cervical and parametrial involvement (4-6).
Pelvic recurrences or symptomatic patients could be considered for palliative RT (4).
HG-ESS/UES treatment
These subtypes, that for similarities and brevity we describe together, represent a small percentage of US. Their prognosis is poor, also due to the low response-rate to systemic therapies (2,15). Three international guidelines (4,7,9) presented their treatment.
Surgery is the only treatment that can impact on patients’ prognosis (total hysterectomy with bilateral salpingo-oophorectomy). Lymphadenectomy is recommended if clinical o radiological suspected lymph nodes are detected (7).
The poor response also to CHT raises relevant doubts about its prescription, though a phase III study showed a positive trend for OS with undifferentiated sarcomas being only a minor subgroup (19). However, the included guidelines suggest adjuvant CHT for all patients (4,7,9).
RT
Like all poorly differentiated tumors, HG-ESS and UES have high local and distant relapse rates. Therefore, RT can be an option in selected high-risk patients: advanced stage or bulky tumors, positive resection margins and deep myometrial invasion (4).
Palliation remains an important field of application for RT in these patients (4).
Conclusions
Our findings show contradictions between the analyzed guidelines probably explained by the rarity and heterogeneity of these neoplasms. Not all histological types were included by all guidelines. Moreover, some of them focused only on one or few USs but with a more extensive discussion (5-8,16). On the contrary several authors described USs within the more general topic of soft tissue sarcomas or of uterine neoplasms, with obvious lack of details (4,9). RT details were not described in the included guidelines. The lack of homogeneity and sometimes the contradictory statements make it difficult to summarize clear suggestions for the daily practice.
However, trying to summarize, we can propose the following observations. All reviewed guidelines exclude RT from primary treatments, in all histological subtypes. In terms of adjuvant therapy, RT is considered in CS as a primary treatment together with CHT by one guideline (5). On the contrary, it is considered only as an optional treatment by two other guidelines (4,8). Similarly, in LM, only one guideline (5) considers RT (and CHT) as a treatment option, while the others suggest RT only in selected cases (4,9,16). In LG-ESS, one guideline excludes RT (9) while the other three only suggest to “consider it” (4-6). Finally, in HG-ESS and UES, one guideline (9) proposes RT as adjuvant therapy (± CHT), while two other guidelines define RT as optional (4,7).
Although cited by some national guidelines (1,13), none of the analyzed international guidelines include neoadjuvant therapy, whether based on RT or systemic treatments.
Finally, only one guideline (4) considers RT as a therapeutic option in the symptomatic treatment of all “true” US (LM, LG-ESS, HG-ESS and UES).
Despite the role of adjuvant RT is not strongly defined in the reviewed guidelines, some recent studies report positive results. The French Sarcoma Group (20) retrospectively described the effect of adjuvant RT and CHT in HG-ESS and UES patients, with improved OS and DFS, despite the small sample size (39 patients in total, 22 patients treated with RT). Moreover, Malouf et al. reported the positive impact of adjuvant RT on OS and PFS in univariate analysis, mainly in locally advanced UES (21).
In summary, in primary therapy, all guidelines exclude RT, regardless of the histological type, both in early and advanced disease, except in those patients where surgery for medical reasons is contraindicated and RT can be taken into account. Considering adjuvant therapy, in no histological type there are uniform indications among the different guidelines regarding RT, moreover, high-risk criteria are not uniformly defined. The use of neoadjuvant therapy, based on RT or systemic therapies, is not considered by any guideline. Surprisingly enough, only one guideline mentions RT as a palliative treatment.
Therefore, the results of our analysis show evident uncertainty about the role of RT in the US. Therefore, there is a clear need for trials evaluating the possible impact of RT in these aggressive tumors. Considering the rarity of these neoplasms, and in particular of some subtypes, randomized studies in this field would require the involvement of several centers. Moreover, as reported also by Ferrandina et al. in an Italian recent review (22), treatment of these patients in referral centers is essential considering the rarity of the disease and the heterogeneity of the available evidence to date. Furthermore, based on the difficulties in carrying out randomized trials in rare diseases such as US, alternative ways of generating scientific evidence in this field should be evaluated. For example, multi-center collaborations with the aim of designing large databases could allow the development of predictive models that can guide the prescription of RT in individual patients with US. Finally, considering the growing role of neoadjuvant therapy in other sarcomas (especially those of the limbs) (23,24), the role of RT should be tested also in this setting.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Gynecology and Pelvic Medicine for the series “Uterine Sarcomas”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-65/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-65/coif). The series “Uterine Sarcomas” was commissioned by the editorial office without any funding or sponsorship. AMP served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Gynecology and Pelvic Medicine from Aug 2020 to Jul 2022. PDI served as the unpaid Guest Editor of the series. CZ reports grants, personal fees and non-financial support from Roche, grants from Eisai, grants, personal fees and non-financial support from Novartis, grants, personal fees and non-financial support from AstraZeneca, grants, personal fees and non-financial support from Pfizer, grants from Pharma PharmaMar, grants and personal fees from Amgen, grants and personal fees from Tesaro, personal fees from QuintikesIMS, grants from Pierre Fabre, grants from Istituto Gentili, grants from Takeda, grants from TEVA, grants from Medivation, grants from Abbvie, grants from Array BioPharma, grants from Morphotek, grants from Synthon, grants from Seattle Genetics, grants from Lilly, grants from Celgene, outside the submitted work. AGM reports grants from Elekta, personal fees from Astellas, personal fees from Alfa-sigma, grants from Tema Sinergie, grants from Janssen, grants from Bayer, grants from Igea, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- AIOM guidelines: Sarcomi dei tessuti molli e GIST. Edizione 2019. Available online: https://www.aiom.it/wp-content/uploads/2019/10/2019_LG_AIOM_Sarcomi-1.pdf (Accessed on 04 Oct 2020).
- Denschlag D, Ackermann S, Battista MJ, et al. Sarcoma of the Uterus. Guideline of the DGGG and OEGGG (S2k Level, AWMF Register Number 015/074, February 2019). Geburtshilfe Frauenheilkd 2019;79:1043-60. [Crossref] [PubMed]
- Kurman RJ, Carcangiu ML, Herrington CS, et al. WHO classification of tumours of female reproductive tract. Lyon: IARC Press, 2014:135-47.
- NCCN Clinical Practice Guidelines in Oncology: Uterine Neoplasms. Version 1.2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (Accessed on 25 Oct 2020).
- PDQ® Adult Treatment Editorial Board. PDQ Uterine Sarcoma Treatment. Bethesda: National Cancer Institute. Updated 19 Nov 2019. Available online: https://www.cancer.gov/types/uterine/hp/uterine-sarcoma-treatment-pdq (Accessed 20 Oct 2020).
- Amant F, Floquet A, Friedlander M, et al. Gynecological cancer intergroup consensus review for endometrial stromal sarcoma. Int J Gynecol Cancer 2014;24:S67-72. [Crossref] [PubMed]
- Pautier P, Nam EJ, Provencher DM, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for high-grade undifferentiated sarcomas of the uterus. Int J Gynecol Cancer 2014;24:S73-7. [Crossref] [PubMed]
- Berton-Rigaud D, Devouassoux-Shisheboran M, Ledermann JA, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for uterine and ovarian carcinosarcoma. Int J Gynecol Cancer 2014;24:S55-60. [Crossref] [PubMed]
- Casali PG, Abecassis N, Aro HT, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv51-67. [Crossref] [PubMed]
- Dangoor A, Seddon B, Gerrand C, et al. UK guidelines for the management of soft tissue sarcomas. Clin Sarcoma Res 2016;6:20. [Crossref] [PubMed]
- Morice P, Rodriguez A, Rey A, et al. Prognostic value of initial surgical procedure for patients with uterine sarcoma: analysis of 123 patients. Eur J Gynaecol Oncol 2003;24:237-40. [PubMed]
- Seidman MA, Oduyebo T, Muto MG, et al. Peritoneal dissemination complicating morcellation of uterine mesenchymal neoplasms. PLoS One 2012;7:e50058. [Crossref] [PubMed]
- Alberta Health Services-Clinical Practice Guideline Gyne-007, version 2. Available online: https://www.albertahealthservices.ca/assets/info/hp/cancer/if-hp-cancer-guide-gyne007-uterine-sarcoma.pdf (Accessed on 12 Oct 2020).
- Lee SW, Lee TS, Hong DG, et al. Practice guidelines for management of uterine corpus cancer in Korea: a Korean Society of Gynecologic Oncology Consensus Statement. J Gynecol Oncol 2017;28:e12. [Crossref] [PubMed]
- Yamagami W, Mikami M, Nagase S, et al. Japan Society of Gynecologic Oncology 2018 guidelines for treatment of uterine body neoplasms. J Gynecol Oncol 2020;31:e18. [Crossref] [PubMed]
- Hensley ML, Barrette BA, Baumann K, et al. Gynecologic Cancer InterGroup (GCIG) consensus review: uterine and ovarian leiomyosarcomas. Int J Gynecol Cancer 2014;24:S61-6. [Crossref] [PubMed]
- Homesley HD, Filiaci V, Markman M, et al. Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a Gynecologic Oncology Group Study. J Clin Oncol 2007;25:526-31. [Crossref] [PubMed]
- Galaal K, Van der Heijden E, Godfrey KN, et al. Adjuvant radiotherapy and/or chemotherapy after surgery for uterine carcinosarcoma. Cochrane Database Syst Rev 2013;2013:CD006812. [Crossref] [PubMed]
- Pautier P, Floquet A, Gladieff L, et al. A randomized clinical trial of adjuvant chemotherapy with doxorubicin, ifosfamide, and cisplatin followed by radiotherapy versus radiotherapy alone in patients with localized uterine sarcomas (SARCGYN study). A study of the French Sarcoma Group. Ann Oncol 2013;24:1099-104. [Crossref] [PubMed]
- Meurer M, Floquet A, Ray-Coquard I, et al. Localized high grade endometrial stromal sarcoma and localized undifferentiated uterine sarcoma: a retrospective series of the French Sarcoma Group. Int J Gynecol Cancer 2019;29:691-8. [Crossref] [PubMed]
- Malouf GG, Lhommé C, Duvillard P, et al. Prognostic factors and outcome of undifferentiated endometrial sarcoma treated by multimodal therapy. Int J Gynaecol Obstet 2013;122:57-61. [Crossref] [PubMed]
- Ferrandina G, Cynthia A, Raimondo BP, et al. Italian consensus conference on management of uterine sarcomas on behalf of S.I.G.O. (Societa' italiana di Ginecologia E Ostetricia). Eur J Cancer 2020;139:149-68. [Crossref] [PubMed]
- Haas RL. Preoperative radiotherapy in soft tissue sarcoma: from general guidelines to personalized medicine. Chin Clin Oncol 2018;7:41. [Crossref] [PubMed]
- Wang D, Zhang Q, Burton L, et al. Significant reduction of late toxicities in patients with extremity sarcoma treated with image-guided radiation therapy to a reduced target volume: results of radiation therapy oncology group RTOG-0630 trial. J Clin Oncol 2015;33:2231-8. [Crossref] [PubMed]
Cite this article as: Ferioli M, Galuppi A, Perrone AM, De Iaco P, Zamagni C, Buwenge M, Morganti AG, Cammelli S. Radiotherapy in uterine sarcoma: a narrative review of international guidelines. Gynecol Pelvic Med 2021;4:17.


