Minimally invasive surgery in pelvic pain: from a gynecological perspective
Pelvic pain
Pelvic pain is defined as pain localized to the pelvis below the umbilicus and usually caused by pelvic organs, as well as pelvic bones, muscles, nerves, joints or blood vessels (1). It can be categorized as acute or chronic. The American College of Obstetrics and Gynecologists defines chronic pelvic pain (CPP) as “pain in the pelvic area that lasts for 6 months or longer and results in functional or psychological disability or requires intervention and treatment” (2,3). CPP can be constant or intermittent. It can be associated with menstruation (dysmenorrhea) or with sexual intercourse (dyspareunia) (4).
According to an extensive review conducted in 2014 the prevalence of noncyclical CPP was reported between 5.7% and 26.6% (5). CPP is the reason for 1 out of 10 gynecological outpatient visits, indication for 15–40% laparoscopies and 12% hysterectomies in the United States (US) (6,7). CPP has a complex pathophysiology, which has not been completely understood. One of the reasons for this complexity is that frequently no underlying pathology can be found. Furthermore, changes in the central nervous system have been identified in CPP patients, which could explain the disparity between the extent of the pathology observed at laparoscopy and the pain perceived by the patient (8,9). Therefore, management of CPP is complicated.
Conditions associated with CPP
A variety of conditions can cause CPP. CPP can be visceral, caused by pelvic organs (bladder, colon, uterus, ovaries, rectum, etc.), can be somatic caused by the musculoskeletal system or can be neuropathic generated by the nerves (2). Furthermore, CPP can be associated with psychosocial disorders such as abuse, depressive disorders, anxiety disorders, somatic disorders and substance use disorders (3). Differential diagnosis of CPP is summarized in Table 1.

Full table
In this review the focus will be on the gynecological and the retroperitoneal pathologies and their minimal invasive surgical treatment from a gynecological and neuropelveological perspective.
Minimally invasive surgery
Under the definition of minimally invasive surgery, surgical procedures, which minimize the surgical incisions and thus expedite wound healing time, decrease post-operative pain and risk of infections, is understood (10). From a gynecological perspective, minimal invasive procedures encompass vaginal approaches such as hysteroscopy and laparoscopic approaches such as conventional laparoscopic and robot-assisted laparoscopic surgery. According to the US surgical data between 1998 and 2010, a decrease in abdominal hysterectomies from 65% to 54% and an increase in minimally invasive techniques have been observed (11).
Vaginal approach: hysteroscopy
Hysteroscopy falls under the category of vaginal techniques in the treatment of CPP. Pathologies such as intrauterine adhesions, polyps, some classes of leiomyomas and to a limited extent adenomyosis can be treated with hysteroscopy. It has been reported that in the presence of leiomyomas of classes 0–2, which cannot be visualized by an abdominal approach but still might be the underlying cause of CPP can be diagnosed and treated with hysteroscopy (12).
Laparoscopic surgery in gynecology
Since the first description of laparoscopic hysterectomy in 1989, laparoscopic surgery has gained in popularity over the last decades and has replaced laparotomy especially in the treatment of benign gynecological conditions despite longer operation times and longer learning curve (13,14). The reason for this increase in laparoscopic surgery is the quicker return of the patients to their normal activities, shorter hospital stays, and fewer wound or abdominal wall infections (14). Furthermore, due to the small incisions, enlarged visual field and the use of powered surgical tools, laparoscopic surgeries are associated with reduced blood loss and decreased postoperative pain (15-17). Laparoscopic surgery can be performed either with the conventional techniques or with the use of a robotic platform.
Diagnostic laparoscopy (DL)
DL provides a total examination of the whole abdominal cavity. According to a study conducted by Howard et al. in 1993, 44% of DLs were performed with an indication of CPP (1). Wiener et al. reported that the primary indication for laparoscopies in UK and the secondary in the US was pelvic pain (18). Back then DL was considered as the gold standard in diagnosis and management of CPP. However, currently with the advance of non-invasive diagnostic and imaging techniques such as 3D ultrasonography, magnetic resonance neurography (MRN) and with a multidisciplinary evaluation, DL has become a second line diagnostic and treatment modality (19). Another challenge with DL arises with the finding of a complicated pathology which is prone to complications. In such situations the surgeons are left with a medicolegal dilemma of taking the risk of causing possible complications and opting for an excision of the pathology perioperatively.
In a retrospective study including 82 CPP patients, in 66% a pathology was identified during DL whereas only 38% received a positive presurgical diagnosis using non-invasive techniques (20). According to a survey conducted by Howard et al. the most common findings in DL were endometriosis and adhesions with a rate of 35% and 24%, respectively (1). Although the findings causing CPP were similar in most of the studies, their percentages differed most probably due to the differences in the patient cohorts (Table 2) (21-25). In addition, approximately in one third of the cases no pathology was detected. However, most of these women stated that going through DL helped them in coping their pain symptoms indicating a psychological involvement associated with laparoscopy (26). Elcombe et al. also observed improvement in pain symptoms following DL even in the absence of a pathology (27).
DL is an invasive procedure and therefore, nowadays it is not routinely performed. It should also be kept in mind that with DL only intraperitoneal pathologies are detectable. Therefore, the presence of possible retroperitoneal pathologies could be missed. Furthermore, in one third of the women with CPP non-gynecological causes should be considered under differential diagnosis before opting for surgery.
Robot-assisted laparoscopic surgery
When compared to conventional laparoscopy, robot-assisted laparoscopic instruments allow a wrist-like motion imitating a human hand and therefore even the basic techniques such as suturing can be more easily learned and performed (28). Furthermore, robotic platform enables a higher quality 3D vision of the surgical field when compared to the conventional laparoscopy (29). Thus, a better visualization of smaller pelvic spaces enables an easy access, better dissection and decreased blood loss (30). However, there is inconsistent data regarding the costs of robot-assisted laparoscopy. Some studies have reported a higher cost in the use of robotic platform (31). Whereas others did not find a significant difference between the costs of conventional laparoscopy and robot-assisted laparoscopy (32,33). Robot-assisted laparoscopy can be applied to all surgeries where a conventional laparoscopy is possible. Especially, robotic platform can be advantageous when operating on deep pelvic spaces such as the nerve entrapment operations in the retroperitoneal region (34). Surgeons such as Lambaudie et al. compared the advances in robot-assisted laparoscopy to the technological evolution of conventional laparoscopy 50 years ago (35).
Surgical interventions in the treatment of CPP
Adhesion surgery
Adhesions are the most frequent pathological findings of DL performed due to CPP (21,23,25) (Figure 1). According to a meta-analysis, adhesions are accountable for 57% of CPP cases (36). Previous abdominal surgeries, pelvic infections and inflammation are among the factors causing pelvic adhesions. Preoperative assessment of adhesions can be tricky, because a physical examination and also advanced imaging techniques might not be helpful in identifying intraabdominal adhesions (37).
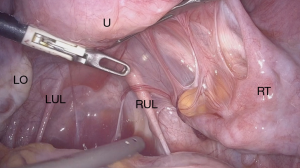
It is still a topic of debate whether these adhesions cause CPP and whether surgery is the treatment of choice. Nezhat et al. explained that pain could be caused by pull on the parietal peritoneum by the adhesions during bowel peristalsis (38). Furthermore, restrictions on the visceral movement through bowel adhesions could be the underlying mechanism (39). On the other hand, Cheong et al. reported that a correlation between the severity, intensity and duration of pain and the localization or type of adhesions visualized during DL did not exist (40). Furthermore, patient anamnesis especially surgical history differentiating between laparoscopy and laparotomy is an essential part of the evaluation. The risk of adhesion formation is higher following a laparotomy than a laparoscopy (41,42).
Several studies support adhesiolysis as an effective treatment of CPP. In a longitudinal study conducted by Nezhat et al., they evaluated pain levels following laparoscopic adhesiolysis in previously hysterectomized patients without any history of endometriosis with a follow-up period of 5 years. They reported pain relief in 72% of the patients postoperatively at 2–8 weeks, in 64% postoperatively at 6–12 months and in 67% postoperatively at 2–5 years (38). Cheong et al. reported a decrease in visual analogue scales (VAS) evaluating pain and an increase in quality of life scores at 6 months follow-up after adhesiolysis (43). Onders et al. performed adhesiolysis in 45 out of 70 patients who received DL due to CPP. Short-term postoperative evaluation yielded a complete relief of pain and at the 6-month follow-up 71% of the patients were still pain free (44).
Although positive effects of adhesiolysis have been reported, the percentage of patients experiencing pain relief varied among studies. In a systematic review, in 18 out of 22 studies representing 92% of the patient population the percentage of pain relief ranged from 56% to 88% (45). A comparison of laparoscopic adhesiolysis with DL at 12-month follow-up in a randomized controlled study yielded no significant differences in VAS scores and quality of life scores of the patients indicating that adhesiolysis had no advantages over DL (46). Due to the randomized design of the study, surgeons were left in a dilemma regarding the efficacy of adhesiolysis. Roman et al. published a critique of this study under the name “Why laparoscopic adhesiolysis should not be the victim of a single randomized clinical trial” where they concluded that the results of this randomized trial were based on a miscalculation of the sample size and statistical power and therefore was insufficient to show the difference between DL and adhesiolysis (47).
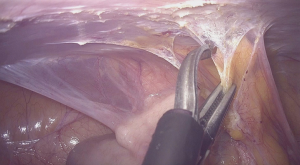
There is no evidence supporting the efficacy of agents for adhesion prevention (41,48). Therefore, to prevent adhesion formation meticulous surgical techniques, which minimize tissue trauma while achieving optimal hemostasis and minimizing the risk of infection are important (49). Gomel et al, reported better results in terms of postoperative adhesion formation, following the application of microsurgical techniques (50). In a study conducted by Luciano et al. where 38 patients underwent laparoscopic adhesiolysis due to moderate to severe adhesions and where they received a second round of laparoscopy 4 weeks after the initial surgery, a significant reduction of the extent and severity of adhesions was observed (51). The preferred method of adhesiolysis especially in the presence of filmy adhesions is dissection with cold scissor (Figure 2). The use of bipolar electrosurgery should be minimal. Ultrasonic scalpel can also be applied on dense adhesions with the advantage of less thermal spread. Although experienced surgeons apply adhesiolysis to filmy adhesions and prefer adhesiectomy to treat dense adhesions, these techniques have not yet been systematically tackled in literature. Studies comparing the efficacy of these two techniques are needed.
Endometriosis surgery
Endometriosis is one of the most common causes of CPP. The disease has a prevalence of 5–21% among women who are hospitalized for pelvic pain (52). On average diagnosis takes about 7 years and during these 7 years patients who are suffering from severe pain seek a solution from a variety of specialists (53).
Endometriosis cause symptoms depending on the organ involvement. Thus, an ovarian endometrioma does not manifest itself same as deep infiltrating endometriosis (DIE). DIE is more severe and depending on the affected organs can be responsible for dysuria, dyschezia in addition the dysmenorrhea, dyspareunia, cyclic or non-cyclic CPP. CPP affects endometriosis patients’ social lives, they fail to attend school or continue their work (54).
One of the most common findings on DL performed with an indication of CPP has been reported as endometriosis (1,21-23). Management of endometriosis related pain can be medical or surgical. Many medical treatments have been implemented and have been effective in suppressing the disease (55). However, none of them are curative. Surgical options can be conservative such as ablation or excision of endometriosis tissue, drainage, excision or sclerosis of endometriomas and excision of bowel nodules. Radical surgery involving hysterectomy with or without bilateral salpingo-oophorectomy (BSO) is also an option (56). In young women who wish to preserve fertility conservative surgery is commonly performed. However, an estimated 50% recurrence has been reported (57). Furthermore, DIE surgery requires a multidisciplinary team with colorectal surgeons and urologist who are also trained in endometriosis surgery. Therefore, ESHRE Guidelines recommend the referral of women with DIE to specialized endometriosis centers where a team of specialists are involved in the management (58).
In a randomized study, Abbott et al. compared DL with laparoscopic excision of endometriosis and reported an 80% improvement in quality of life and VAS scores at 6th month following endometriosis surgery (59). Cochrane review also reported the benefits of endometriosis surgery over DL (60). In a multicenter prospective study conducted at 51 certificated endometriosis centers with 4,721 patients, a significant reduction in VAS scores assessing premenstrual pain, menstrual pain and non-cyclic pelvic pain and a significant improvement in quality of life scores at 6th month following laparoscopic excision of deep endometriosis was reported (61).
Minimally invasive surgery, both conventional laparoscopy and robot-assisted laparoscopy, is beneficial in endometriosis-related pain. However, additional postoperative medical treatment such as gonadotropin-releasing hormone (GnRH) analogues, progestins or levonorgestrel intrauterine devices (LNG-IUD) is needed to reduce recurrence and alleviate pain (62).
Adenomyosis surgery
Adenomyosis triggers chronic inflammation in the myometrium through diffuse or focal invasion, and this inflammation is responsible for the pain (63). Incidence varies depending on age and ethnicity (64). In 25–65% of hysterectomy specimen adenomyosis was reported histopathologically (65). In the past, the reason for this high histopathological diagnosis following hysterectomy was due to the insufficiency in preoperative diagnosis. However, today ultrasonographic findings of adenomyosis are well described (66). Studies show that adenomyosis can be identified with high sensitivity and specificity using transvaginal ultrasonography (67,68). In addition, magnetic resonance imaging (MRI) can also be used in the preoperative diagnosis (69,70). Although medical treatment such as nonsteroidal anti-inflammatory drugs (NSAIDs), GnRH analogues, LNG-IUD and uterine artery embolization can be used in the treatment, gold standard is hysterectomy (71).
Adenomyosis surgery involves either a complete excision of the disease called adenomyomectomy or a cytoreductive surgery called partial adenomyomectomy (72). Kwack et al. compared 108 laparoscopic and 116 laparotomic adenomyomectomies and observed a significant alleviation in symptoms (73). The authors recommended laparotomic approach in diffuse adenomyosis and laparoscopy in focal adenomyosis.
Differentiation between adenomyotic tissue and the surrounding healthy myometrium issue is not always possible. Therefore, dissection can be difficult (74). For this reason, a standard surgical technique in uterus sparing surgery for patients with a fertility wish does not exist. Several studies have reported the application of uterus sparing surgical techniques in adenomyosis surgery (74-77). Osada et al. reported complete resection of diffuse adenomyosis with laparotomy and showed a significant reduction in pain. Furthermore, during the long-term follow-up, 58% of these patients gave birth to healthy infants (74). Saremi et al. observed a significant relief in dysmenorrhea following adenomyomectomy in 41% of their patients (75). Takeuchi et al. performed laparoscopic adenomyomectomy on 14 patients and reported significant decrease in the VAS scores during the follow-up (77). Huang et al. compared two laparoscopic techniques, which were classic/three-flap technique and double-flap technique and concluded that double-flap technique was more easily performed and more effective in the dissection of diffuse adenomyosis (76).
Chong et al. performed laparoscopic adenomyomectomy in 25 patients with conventional techniques and in 8 patients with robot-assisted laparoscopy and stated the feasibility of minimally invasive surgical options in uterus sparing surgeries (78). These findings were supported by Chung et al. (79). A study comparing robot-assisted laparoscopic adenomyomectomy with laparoscopic adenomyomectomy reported no significant differences between the two methods (80). Although these studies were not conclusive on the advantages of robot-assisted laparoscopy, the advance mobility of robotic instruments and higher quality of 3D vision is likely to be advantageous in adenomyosis tissue excision and suturing. However, further studies are needed to support these hypotheses.
Myomectomy
Whether leiomyomas cause CPP is dependent on their localization, size, number and if they are degenerated or not. The most common symptom of leiomyomas is abnormal uterine bleeding. This abnormal bleeding causes uterine contraction, which causes cyclic pelvic pain (69). In addition, large leiomyomas can compromise neighboring structures resulting in pain.
In comparison to other pathologies leiomyomas rarely cause CPP. Therefore, a thorough examination is recommended to exclude other pathologies (81). However, if a decision is made for myomectomy, conventional laparoscopy or robot-assisted laparoscopy should be the choice of surgical technique (65). In addition, a comparison between laparoscopic myomectomy and myomectomy with mini-laparotomy, also showed the superiority of laparoscopy in terms of postoperative analgesia (82).
Hysterectomy
Hysterectomy is one of the most common gynecological surgeries performed. Approximately 12% of hysterectomies in the US are performed for CPP (83). It is also the wish of many patients who suffer from CPP due to the notion that uterus is the sole reason for their pain. However, its place in the treatment of CPP is still uncertain. According to a retrospective analysis of 9,622 patients who underwent open or laparoscopic hysterectomies for benign conditions, 40% of the patients were operated with an indication of CPP (84). The high rate reported in this study was due to the exclusion of vaginal hysterectomies, which is the preferred surgical technique in patients with descensus uteri. Hysterectomy is most commonly offered to CPP patients who are in their perimenopausal period or who have no fertility desires.
Before offering a patient hysterectomy as a treatment option, it should be kept in mind that in 40% pain symptoms could persist postoperatively and in 5% pain could worsen (85). Brandsborg et al. reported in 32% of the cases postoperative continuation of CPP during a 15-month follow-up (86). On the other hand, Hillis et al. reported a total recovery in 74%, a decrease in pain symptoms in 21% and persistence of CPP in 5%. They also evaluated CPP in specific subgroups and observed that in the long-term 40% of the patients continued to suffer from CPP (87). In a larger prospective cohort study Hartmann et al. compared postoperatively at 6th and 24th months the quality of life scores and sexual function of CPP patients who presented preoperatively with only CPP or with CPP and depression. According to the results of this study, patients who suffered both from CPP and depression prior to their hysterectomies had poorer results during the 24-month follow-up when compared to patients suffering from either disorder alone or neither. However, all patients including the ones suffering from both disorders showed an improvement in their quality of life scores and their evaluation of sexual function following hysterectomy (88).
In order to decrease the high rates of postoperative persistence of CPP, these patients should be evaluated thoroughly prior to hysterectomy with a multidisciplinary team. Gastrointestinal, genitourinary, musculoskeletal and psychiatric evaluation could reveal other underlying pathologies (49). In addition, a neuropelveological examination is also necessary to exclude pelvic retroperitoneal pathologies with neural involvement. It has been reported that women who are hysterectomized without a clear preoperative pathological finding benefit less from the surgery (89). However, it is also common in CPP patients that despite thorough preoperative examination an underlying pathology cannot be found as mentioned in DL section.
Following the preoperative evaluation, if hysterectomy is the choice of treatment the operation should be performed with minimal invasive techniques. It is not certain if addition of oophorectomy to the operation is more beneficial on pain (85,90). However, if oophorectomy is also performed, a total excision of ovarian tissue should be aimed in order to avoid ovarian remnant syndrome (ORS) and cause a new pain etiology.
ORS
In patients with a history of BSO with or without hysterectomy presenting with CPP, ORS should come to mind. According to Behera et al., 26% of patients who underwent BSO with or without hysterectomy have been diagnosed with ORS (25). ORS occurs following an incomplete excision of ovarian tissue, which results in development of a pelvic mass causing CPP.
Previous abdominal surgeries, pelvic infections, endometriosis, dense adhesions, inflammatory bowel syndrome, ruptured appendicitis, leaving ovarian tissue in pelvis because of an intraoperative complication or inability to ligate ovarian tissue with a safe distance can lead to incomplete removal of ovarian tissue (91). If a patient does not enter surgical menopause following BSO, ORS should be suspected.
Excision of the remnant tissue is the treatment of choice. However, prior to excision iliac vessels and ureters should be dissected and dense bowel adhesions should be removed. Nezhat et al. described a hydro-dissection technique with a laparoscopic approach (92). Following a complete excision of the remnant ovarian tissue, a complete resolution of CPP or significant relief has been reported (93). In order to avoid ORS a sharp dissection instead of a blunt dissection of the adhesions between the ovaries and the adjacent structures has been recommended (91).
Neurolysis and pelvic denervation procedures
In order to evaluate retroperitoneal neural involvement in the differential diagnosis of CPP, a comprehensive knowledge on pelvic autonomic and somatic nervous system and their projections on muscles and dermatomes as a functional unit is required. Without a complete understanding of pelvic neurophysiology, it is impossible to conclude whether a nerve pathology is the underlying etiology of CPP. Also, orthopedic and/or spinal pathologies should be excluded before searching for a pelvic nerve pathology.
The neural evaluation starts with a correct definition of visceral and somatic pain. Visceral component of CPP requires a thorough understanding of the pelvic autonomic innervation. Visceral pain is diffuse, not well localized and it is conducted by the hypogastric plexus. Para-aortic sympathetic trunk forms the superior hypogastric plexus. Neurons descending down bilaterally join the pelvic splanchnic nerves to form the inferior hypogastric plexus. Both sympathetic and parasympathetic fibers of the hypogastric plexus are responsible for conduction of the nociceptive signals from the pelvic organs (94). Due to this autonomic innervation, visceral pain is also associated with symptoms such as malaise, bloating, nausea, vomiting and syncope. On the other hand, somatic pain is conducted by the lumbosacral plexus (L4–5, S1–5) and pathologies affecting the plexus or the somatic nerves origination from the plexus are responsible for well localized pain or loss of sensation at the lower abdominal wall, perineal region and lower extremities (95) (Figure 3).
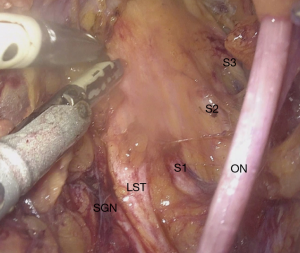
In addition to a thorough anamnesis neurological examination also involves MRN and/or electromyography (EMG). A thorough surgical history has also utmost importance, because of possible nerve injuries. For instance, lateral trocar entrance areas proximal to the spina iliaca anterior superior are also where the ilioinguinal and iliohypogastric nerves originating from the lumbosacral plexus enter anterior abdominal wall. An injury sustained by the entrance of trocars during laparoscopy, can results in anterior abdominal wall pain. These nerves can also be injured during a herniation repair or by operations where a wide transverse skin incision is required (96). In addition, genitofemoral nerve is located on the psoas muscle and can easily be damaged during abdominal surgery (97) (Figure 4).
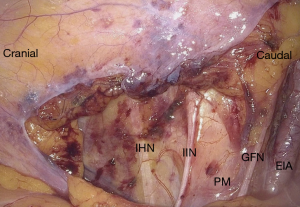
Several minimal invasive procedures involving pelvic nerves have been described in literature. Two of them, laparoscopic uterosacral nerve ablation (LUNA) and presacral neurectomy (PSN) are categorized under pelvic denervation. Whereas a newer approach called neuropelveology focuses on nerve sparing techniques also known as neurolysis (95).
LUNA
LUNA is a procedure where uterosacral ligaments are transected close to their insertion into the cervix. This leads to the disruption of afferent sensory nerve fibers of the Frankenhauser plexus, which are responsible for pain originating in uterus, cervix and other pelvic structures. Before the introduction of laparoscopy, this procedure was performed by either a vaginal or an abdominal approach (98). Currently, LUNA is performed by laparoscopy using laser or bipolar electrosurgery followed by transection of the ligaments with cold scissors.
When LUNA was first introduced to practice, it was widely accepted. However, as the knowledge of pelvic neural anatomy advanced, the idea of nerve transection to treat pain became absurd. From a neuropelveological perspective anatomical disruption of nerve fibers does not have a place in pain treatment. This realization was also supported through several studies, which showed that LUNA was ineffective in the treatment of CPP, dysmenorrhea, dyspareunia and did not have a positive effect on quality of life (99,100). Furthermore, in a double blinded study conducted by Johnson et al. on 123 women, LUNA was found to be ineffective on non-menstrual pain, dyspareunia and dyschezia regardless of the presence of endometriosis. The authors concluded that neuroablative surgeries like LUNA could only be successful if all the afferent nerves from all pelvic organs were to be transected. Since such a procedure was unrealistic, the authors pointed out that neuroablative surgery was not suitable for CPP treatment (101).
PSN
PSN is another pelvic denervation procedure where the afferent presacral nerve fibers are transected at the superior hypogastric plexus. When compared to LUNA in patients with primary dysmenorrhea, PSN was reported to be more effective in the long-term treatment (102).
PSN is performed mostly in addition to a surgical procedure to enhance pain treatment. In a study conducted by Nezhat et al. in 75% of the patients with endometriosis who underwent endometriosis surgery with PSN, postoperative pain was significantly reduced (103). However, since this study did not include a control group, it was not conclusive whether the reduction in pain was due to PSN or endometriosis surgery. In a randomized controlled study with 71 moderate to severe endometriosis patients conservative endometriosis surgery and conservative endometriosis surgery with PSN were applied (104). During a 12-month follow-up no significant difference between the groups in terms of dysmenorrhea, CPP and dyspareunia were observed. A slight reduction in the midline component of menstrual pain was reported however this reduction was not statistically significant. Zullo et al. designed a double-blind randomized controlled study with 141 patients with endometriosis who underwent surgery with or without PSN. At 6th and 12th month follow-up examinations significant improvement in dysmenorrhea, dyspareunia and CPP were observed in patients who had received PSN (105).
Due to the localization of the superior hypogastric plexus below the level of the aortic bifurcation between the common iliac vessel at the interiliac triangle, which is surrounded densely by vessels and nerves, this procedure is prone to complications related to injury (106). In addition, complications such as chylous ascites, urinary retention, small bowel obstruction, painless labor, vaginal dryness and sexual dysfunction have also been reported (107). The most common complication following PSN was constipation observed in 74% of the cases (108).
According to literature the effectiveness of PSN in treatment of CPP is still inconclusive. Similar to the reasoning with LUNA, nerve transection should not be a choice of treatment without clarifying the etiology of pain. With the current advances in minimal invasive surgery nerve sparing procedures are more commonly performed. However, PSN could still be used in the treatment of midline pain where other treatment options have failed. More randomized controlled studies with larger cohorts are needed to evaluate PSN effects on treatment of midline pain (109).
Neurolysis
With the introduction of laparoscopic neuronavigation (LANN), first described by Possover et al., the visualization of retroperitoneal area with laparoscopy and the dissection of autonomic and somatic nerves under microscopic view became possible (110). Following this visualization of the lumbosacral plexus, nerve pathologies such as entrapment or direct involvement of the nerve fibers could be identified (94,111).
It has been shown with LANN that endometriosis is responsible for all the pathologies involving nerve fibers in the retroperitoneal area. According to Siquara De Sousa et al. in the presence of retroperitoneal endometriosis a 57% lumbosacral plexus involvement and a 39% sciatic nerve involvement has been reported (112). Possover et al. have shown that in the treatment of neural involvement of endometriosis, resection of endometrial tissue present within the nerve is possible without causing any neural complications (111). Endometriosis can also infiltrate into the sacral nerve roots. Sympathetic fibers are more affected than the somatic fibers, because the hypogastric fascia acts as a barrier against endometriosis infiltration (113). In 40% of patients with DIE and in 72% of patients with hypogastric endometriosis deep lateral pelvic endometriosis has also been observed (114). CPP was also reported as the main symptom and indicator of deep later pelvic endometriosis (114). Possover et al. reported that neurolysis of the sacral nerve roots is sufficient and unchallenging in the treatment of endometriosis involving sacral nerves since endometriosis does not infiltrate into the epineurium (111,113).
The second pathology which can be treated with the neuropelveological approach is the entrapment of a nerve. Entrapment can be caused by endometriosis, vascular malformations/dilatations, fibrosis and piriformis syndrome where the piriformis muscle fibers entrap the sciatic nerve (94). Previous surgeries, especially pelvic reconstructive surgery have been reported to be responsible for fibrosis in the retroperitoneal area (25,115). Laparoscopy should again be the first line treatment of neural entrapment especially in treating fibrosis following pelvic surgery (116).
Knowledge on neuropelveology enabled surgeons to look for causes of CPP in the retroperitoneal area when DL revealed no findings. A joint evaluation done by a well-trained radiologist and surgeon can lead to a better preoperative diagnosis. Retroperitoneal area, a risky area due to the dense localization of vessels and nerves can be dissected without causing any complications by a surgeon who is trained in anatomy and neuropelveology leading to full recovery with low morbidity (111,114)
Vascular entrapment
Vascular abnormalities can also be the cause of nerve entrapment (Figure 5). However, due to the lack of knowledge on this condition by the clinicians and misdiagnosis of these patients, the actual prevalence is unknown. Patients suffer from severe menstrual pain, an increase in CPP during the day associated with the time a patient spends on her feet and an increase in symptoms during pregnancy.
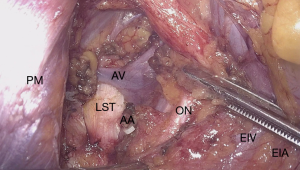
Its etiology can be explained by dilated or malformed branches of the iliac vessels, most likely originating from the iliac vein, which can entrap the nerves of the sacral plexus against the bony structures of the pelvis resulting in above-mentioned symptoms. Furthermore, this condition can present itself in addition to CPP with sciatica, perineal pain, refractory urinary and defecatory dysfunctions (111).
Patients presenting with these symptoms should be examined both from a gynecological and from a neuropelveological perspective. Standard MRI might not be enough to determine the pathology. A radiologist trained in detecting these vascular malformations are vital for the pre-operative evaluation (117).
Possover et al. reported in a series including 97 patients 3 common localizations of entrapment. According to their study, in 62 patients, after entering the sacral foramen, nerves originating from the S2–4 sacral roots are entrapped between piriformis muscle and hypogastric fascia. In 24 patients pudendal nerve is entrapped at the lesser sciatic notch, and in 11 patients sciatic nerve is entrapped before entering the greater sciatic notch between linea terminalis and dilated vessels (118). In addition, they also reported that pain symptoms were associated with the localization of entrapment.
From a neuropelveological perspective the treatment of vascular entrapment encompasses the entrance of lumbosacral fossa either with laparoscopy or with robot-assisted laparoscopy and coagulation and ligation of the malformed or dilated vessels (34,119). An experienced surgeon familiar with the pelvic anatomy and with a thorough pre-operative evaluation can perform this decompression surgery without any complications (118).
Pelvic congestion syndrome (PCS)
PCS should come to mind as differential diagnosis of CPP especially in women of premenopausal age. An increase in pain during the day and before menstruation and pain following sexual intercourse are associated with PCS. In addition, presence of varicose veins in vulva and lower extremities can hint to the presence of PCS (120). Actual incidence is unknown. Kang et al. reported that in 13% of DL performed for CPP, PCS was the diagnosis (22). DL is not recommended in PCS cases, because the dilated pelvic veins collapse due to the Trendelenburg positioning of the operating table. If PCS is suspected during DL pelvic vessels should be evaluated after the elevation of the patient’s head (121). Venography is the gold standard in diagnosis. If medical treatment is not successful, ligation of varicose veins or hysterectomy with BSO can be performed (122,123). Embolotherapy is also a successful treatment option (124).
Conclusions
Due to the multifactorial etiology of CPP, its diagnosis and management is challenging in clinical practice. CPP can be caused by gynecological, gastrointestinal, urological, musculoskeletal and psychological conditions. Therefore, a multidisciplinary management and if possible in specialized centers is recommended. Nowadays, with the advances in minimal invasive surgical techniques and with the introduction of new approaches such as neuropelveology more patients receive a successful diagnosis and treatment. Even in complicated operations such as DIE surgeries, both conventional laparoscopy and robot-assisted laparoscopy have replaced laparotomy. As the understanding of pelvic neurophysiology advances nerve sparing surgeries with minimal invasive techniques are replacing neuroablative procedures with better outcomes. Thus, minimal invasive surgery is now the gold standard in surgical treatment of CPP. However, despite of all these advances recurrence and persistence of pain postoperatively should always be kept in mind. Therefore, a multidisciplinary preoperative evaluation is always emphasized for better outcomes. In Table 3, the take-home messages of this review can be seen.

Full table
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Gokhan Kilic) for the series “Minimally Invasive Treatment Modalities for Female Pelvic Floor Disorders” published in Gynecology and Pelvic Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm-2020-pfd-04/coif). The series “Minimally Invasive Treatment Modalities for Female Pelvic Floor Disorders” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Howard FM. The role of laparoscopy in chronic pelvic pain: promise and pitfalls. Obstet Gynecol Surv 1993;48:357-87. [Crossref] [PubMed]
- Smith SE, Eckert JM. Interventional Pain Management and Female Pelvic Pain: Considerations for Diagnosis and Treatment. Semin Reprod Med 2018;36:159-63. [Crossref] [PubMed]
- Chronic Pelvic Pain. ACOG Practice Bulletin, Number 218. Obstet Gynecol 2020;135:e98-109. [Crossref] [PubMed]
- Chronic Pelvic Pain [Internet]. [cited 2020 May 6]. Available online: Pelvic Painhttps://www.acog.org/en/Patient Resources/FAQs/Gynecologic Problems/Chronic
- Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Physician 2014;17:E141-7. [PubMed]
- Jarrell JF, Vilos GA, Allaire C, et al. No. 164-Consensus Guidelines for the Management of Chronic Pelvic Pain. J Obstet Gynaecol Can 2018;40:e747-87. [Crossref] [PubMed]
- Reiter RC. A profile of women with chronic pelvic pain. Clin Obstet Gynecol 1990;33:130-6. [Crossref] [PubMed]
- Vercellini P, Fedele L, Aimi G, et al. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod 2007;22:266-71. [Crossref] [PubMed]
- Brawn J, Morotti M, Zondervan KT, et al. Central changes associated with chronic pelvic pain and endometriosis. Hum Reprod Update 2014;20:737-47. [Crossref] [PubMed]
- Truong M, Kim JH, Scheib S, et al. Advantages of robotics in benign gynecologic surgery. Curr Opin Obstet Gynecol 2016;28:304-10. [Crossref] [PubMed]
- Committee on Gynecologic Practice. Committee Opinion No 701: Choosing the Route of Hysterectomy for Benign Disease. Obstet Gynecol 2017;129:e155-9. [Crossref] [PubMed]
- Di Spiezio Sardo A, Guida M, Bettocchi S, et al. Role of hysteroscopy in evaluating chronic pelvic pain. Fertil Steril 2008;90:1191-6. [Crossref] [PubMed]
- Reich H, DeCaprio J, McGlynn F. Laparoscopic Hysterectomy. J Gynecol Surg 1989;5:213-6. [Crossref]
- Aarts JW, Nieboer TE, Johnson N, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev 2015;2015:CD003677. [Crossref] [PubMed]
- Nagata H, Komatsu H, Nagaya Y, et al. Comparison of Total Laparoscopic Hysterectomy with Abdominal Total Hysterectomy in Patients with Benign Disease: A Retrospective Cohort Study. Yonago Acta Med 2019;62:273-7. [Crossref] [PubMed]
- Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: A systematic review. J Minim Access Surg 2012;8:67-73. [Crossref] [PubMed]
- Kluivers KB, Hendriks JCM, Mol BWJ, et al. Quality of life and surgical outcome after total laparoscopic hysterectomy versus total abdominal hysterectomy for benign disease: a randomized, controlled trial. J Minim Invasive Gynecol 2007;14:145-52. [Crossref] [PubMed]
- Wiener J. Chronic pelvic pain. Practitioner 1994;238:352-7. [PubMed]
- Royal College of Obstetricians and Gynecologists. Chronic Pelvic Pain, Initial Management (Green-top Guideline No. 41). Royal College of Obstetricians and Gynaecologists [Internet]. 2017 [cited 2020 May 8]; Green-top Guideline (No. 41). Available online: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg41/
- Hebbar S, Chawla C. Role of laparoscopy in evaluation of chronic pelvic pain. J Minim Access Surg 2005;1:116-20. [Crossref] [PubMed]
- Drozgyik I, Vizer M, Szabó I. Significance of laparoscopy in the management of chronic pelvic pain. Eur J Obstet Gynecol Reprod Biol 2007;133:223-6. [Crossref] [PubMed]
- Kang SB, Chung HH, Lee HP, et al. Impact of diagnostic laparoscopy on the management of chronic pelvic pain. Surg Endosc 2007;21:916-9. [Crossref] [PubMed]
- Doyle DF, Li TC, Richmond MN. The prevalence of continuing chronic pelvic pain following a negative laparoscopy. J Obstet Gynaecol 1998;18:252-5. [Crossref] [PubMed]
- Milingos S, Protopapas A, Kallipolitis G, et al. Laparoscopic evaluation of infertile patients with chronic pelvic pain. Reprod Biomed Online 2006;12:347-53. [Crossref] [PubMed]
- Behera M, Vilos GA, Hollett-Caines J, et al. Laparoscopic findings, histopathologic evaluation, and clinical outcomes in women with chronic pelvic pain after hysterectomy and bilateral salpingo-oophorectomy. J Minim Invasive Gynecol 2006;13:431-5. [Crossref] [PubMed]
- Yasmin H, Bombieri L, Hollingworth J. What happens to women with chronic pelvic pain after a negative [normal] laparoscopy? J Obstet Gynaecol 2005;25:283-5. [Crossref] [PubMed]
- Elcombe S, Gath D, Day A. The psychological effects of laparoscopy on women with chronic pelvic pain. Psychol Med 1997;27:1041-50. [Crossref] [PubMed]
- Møller SG, Dohrn N, Brisling SK, et al. Laparoscopic Versus Robotic-assisted Suturing Performance Among Novice Surgeons: A Blinded, Cross-Over Study. Surg Laparosc Endosc Percutan Tech 2020;30:117-22. [Crossref] [PubMed]
- Lawrie TA, Liu H, Lu D, et al. Robot-assisted surgery in gynaecology. Cochrane Database Syst Rev 2019;4:CD011422. [PubMed]
- Boggess JF. Robotic surgery in gynecologic oncology: evolution of a new surgical paradigm. J Robot Surg 2007;1:31-7. [Crossref] [PubMed]
- van Dam P, Hauspy J, Verkinderen L, et al. Are costs of robot-assisted surgery warranted for gynecological procedures? Obstet Gynecol Int 2011;2011:973830. [Crossref] [PubMed]
- Winter ML, Leu SY, Lagrew DC, et al. Cost comparison of robotic-assisted laparoscopic hysterectomy versus standard laparoscopic hysterectomy. J Robot Surg 2015;9:269-75. [Crossref] [PubMed]
- Lönnerfors C, Reynisson P, Persson J. A randomized trial comparing vaginal and laparoscopic hysterectomy vs robot-assisted hysterectomy. J Minim Invasive Gynecol 2015;22:78-86. [Crossref] [PubMed]
- Taner U, Aysel O, Ahmet K, et al. Robot-assisted laparoscopic management of a vascular entrapment of the sacral nerve roots causing pelvic pain. J Obstet Gynaecol Res 2019;45:1603-7. [Crossref] [PubMed]
- Lambaudie E, Houvenaeghel G, Walz J, et al. Robot-assisted laparoscopy in gynecologic oncology. Surg Endosc 2008;22:2743-7. [Crossref] [PubMed]
- ten Broek RP, Issa Y, van Santbrink EJ, et al. Burden of adhesions in abdominal and pelvic surgery: systematic review and met-analysis. BMJ 2013;347:f5588. [Crossref] [PubMed]
- Cunanan RG, Courey NG, Lippes J. Laparoscopic findings in patients with pelvic pain. Am J Obstet Gynecol 1983;146:589-91. [Crossref] [PubMed]
- Nezhat FR, Crystal RA, Nezhat CH, et al. Laparoscopic Adhesiolysis and Relief of Chronic Pelvic Pain. JSLS 2000;4:281-5. [PubMed]
- Kresch AJ, Seifer DB, Sachs LB, et al. Laparoscopy in 100 women with chronic pelvic pain. Obstet Gynecol 1984;64:672-4. [PubMed]
- Cheong Y, Saran M, Hounslow JW, et al. Are pelvic adhesions associated with pain, physical, emotional and functional characteristics of women presenting with chronic pelvic pain? A cluster analysis. BMC Womens Health 2018;18:11. [Crossref] [PubMed]
- Kavic SM, Kavic SM. Adhesions and Adhesiolysis: The Role of Laparoscopy. JSLS 2002;6:99-109. [PubMed]
- Maier DB, Nulsen JC, Klock A, et al. Laser laparoscopy versus laparotomy in lysis of pelvic adhesions. J Reprod Med 1992;37:965-8. [PubMed]
- Cheong YC, Reading I, Bailey S, et al. Should women with chronic pelvic pain have adhesiolysis? BMC Womens Health 2014;14:36. [Crossref] [PubMed]
- Onders RP, Mittendorf EA. Utility of laparoscopy in chronic abdominal pain. Surgery 2003;134:549-52; discussion 552-4. [Crossref] [PubMed]
- Gerner-Rasmussen J, Burcharth J, Gögenur I. The efficacy of adhesiolysis on chronic abdominal pain: a systematic review. Langenbecks Arch Surg 2015;400:567-76. [Crossref] [PubMed]
- Swank DJ, Swank-Bordewijk SCG, Hop WCJ, et al. Laparoscopic adhesiolysis in patients with chronic abdominal pain: a blinded randomised controlled multi-centre trial. Lancet 2003;361:1247-51. [Crossref] [PubMed]
- Roman H, Hulsey TF, Marpeau L, et al. Why laparoscopic adhesiolysis should not be the victim of a single randomized clinical trial. Am J Obstet Gynecol 2009;200:136.e1-136.e4. [Crossref] [PubMed]
- Vettoretto N, Carrara A, Corradi A, et al. Laparoscopic adhesiolysis: consensus conference guidelines. Colorectal Dis 2012;14:e208-15. [Crossref] [PubMed]
- Holloran-Schwartz MB. Surgical evaluation and treatment of the patient with chronic pelvic pain. Obstet Gynecol Clin North Am 2014;41:357-69. [Crossref] [PubMed]
- Gomel V, Koninckx PR. Microsurgical principles and postoperative adhesions: from the past. Fertil Steril 2016;106:1025-31. [Crossref] [PubMed]
- Luciano DE, Roy G, Luciano AA. Adhesion reformation after laparoscopic adhesiolysis: where, what type, and in whom they are most likely to recur. J Minim Invasive Gynecol 2008;15:44-8. [Crossref] [PubMed]
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med 2020;382:1244-56. [Crossref] [PubMed]
- Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2011;96:366-373.e8. [Crossref] [PubMed]
- De Graaff AA, D'Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod 2013;28:2677-85. [Crossref] [PubMed]
- Luciano DE, Luciano AA. Management of endometriosis-related pain: an update. Womens Health Lond Engl 2011;7:585-90. [Crossref] [PubMed]
- Frishman GN, Salak JR. Conservative surgical management of endometriosis in women with pelvic pain. J Minim Invasive Gynecol 2006;13:546-58. [Crossref] [PubMed]
- Vercellini P, Crosignani PG, Abbiati A, et al. The effect of surgery for symptomatic endometriosis: the other side of the story. Hum Reprod Update 2009;15:177-88. [Crossref] [PubMed]
- Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod 2014;29:400-12. [Crossref] [PubMed]
- Abbott J, Hawe J, Hunter D, et al. Laparoscopic excision of endometriosis: a randomized, placebo-controlled trial. Fertil Steril 2004;82:878-84. [Crossref] [PubMed]
- Duffy JMN, Arambage K, Correa FJS, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev 2014.CD011031. [PubMed]
- Byrne D, Curnow T, Smith P, et al. Laparoscopic excision of deep rectovaginal endometriosis in BSGE endometriosis centres: a multicentre prospective cohort study. BMJ Open 2018;8:e018924. [Crossref] [PubMed]
- Yeung PP, Shwayder J, Pasic RP. Laparoscopic management of endometriosis: comprehensive review of best evidence. J Minim Invasive Gynecol 2009;16:269-81. [Crossref] [PubMed]
- Senapati S, Atashroo D, Carey E, et al. Surgical interventions for chronic pelvic pain. Curr Opin Obstet Gynecol 2016;28:290-6. [Crossref] [PubMed]
- Yu O, Schulze-Rath R, Grafton J, et al. Adenomyosis incidence, prevalence and treatment: United States population-based study 2006-201. Am J Obstet Gynecol 2020;223:94.e1-94.e10. [Crossref]
- Solnik MJ, Munro MG. Indications and alternatives to hysterectomy. Clin Obstet Gynecol 2014;57:14-42. [Crossref] [PubMed]
- Van den Bosch T, Dueholm M, Leone FP, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol 2015;46:284-98. [Crossref] [PubMed]
- Rasmussen CK, Hansen ES, Ernst E, et al. Two- and three-dimensional transvaginal ultrasonography for diagnosis of adenomyosis of the inner myometrium. Reprod Biomed Online 2019;38:750-60. [Crossref] [PubMed]
- Exacoustos C, Brienza L, Di Giovanni A, et al. Adenomyosis: three-dimensional sonographic findings of the junctional zone and correlation with histology. Ultrasound Obstet Gynecol 2011;37:471-9. [Crossref] [PubMed]
- Won HR, Abbott J. Optimal management of chronic cyclical pelvic pain: an evidence-based and pragmatic approach. Int J Womens Health 2010;2:263-77. [PubMed]
- Kunz G, Beil D, Huppert P, et al. Adenomyosis in endometriosis--prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum. Reprod 2005;20:2309-16. [Crossref] [PubMed]
- Oliveira MAP, Crispi CP, Brollo LC, et al. Surgery in adenomyosis. Arch Gynecol Obstet 2018;297:581-9. [Crossref] [PubMed]
- Grimbizis GF, Mikos T, Tarlatzis B. Uterus-sparing operative treatment for adenomyosis. Fertil Steril 2014;101:472-87. [Crossref] [PubMed]
- Kwack JY, Im KS, Kwon YS. Conservative surgery of uterine adenomyosis via laparoscopic versus laparotomic approach in a single institution. J Obstet Gynaecol Res 2018;44:1268-73. [Crossref] [PubMed]
- Osada H, Silber S, Kakinuma T, et al. Surgical procedure to conserve the uterus for future pregnancy in patients suffering from massive adenomyosis. Reprod Biomed Online 2011;22:94-9. [Crossref] [PubMed]
- Saremi A, Bahrami H, Salehian P, et al. Treatment of adenomyomectomy in women with severe uterine adenomyosis using a novel technique. Reprod Biomed Online 2014;28:753-60. [Crossref] [PubMed]
- Huang X, Huang Q, Chen S, et al. Efficacy of laparoscopic adenomyomectomy using double-flap method for diffuse uterine adenomyosis. BMC Womens Health 2015;15:24. [Crossref] [PubMed]
- Takeuchi H, Kitade M, Kikuchi I, et al. Laparoscopic adenomyomectomy and hysteroplasty: a novel method. J Minim Invasive Gynecol 2006;13:150-4. [Crossref] [PubMed]
- Chong GO, Lee YH, Hong DG, et al. Long-Term Efficacy of Laparoscopic or Robotic Adenomyomectomy with or without Medical Treatment for Severely Symptomatic Adenomyosis. Gynecol Obstet Invest 2016;81:346-52. [Crossref] [PubMed]
- Chung YJ, Kang SY, Choi MR, et al. Robot-Assisted Laparoscopic Adenomyomectomy for Patients Who Want to Preserve Fertility. Yonsei Med J 2016;57:1531-4. [Crossref] [PubMed]
- Shim JI, Jo EH, Kim M, et al. A comparison of surgical outcomes between robot and laparoscopy-assisted adenomyomectomy. Medicine (Baltimore) 2019;98:e15466. [Crossref] [PubMed]
- Carter JE. Surgical treatment for chronic pelvic pain. JSLS 1998;2:129-39. [PubMed]
- Palomba S, Zupi E, Falbo A, et al. A multicenter randomized, controlled study comparing laparoscopic versus minilaparotomic myomectomy: reproductive outcomes. Fertil Steril 2007;88:933-41. [Crossref] [PubMed]
- Wu JM, Wechter ME, Geller EJ, et al. Hysterectomy rates in the United States, 2003. Obstet Gynecol 2007;110:1091-5. [Crossref] [PubMed]
- Mowers EL, Lim CS, Skinner B, et al. Prevalence of Endometriosis During Abdominal or Laparoscopic Hysterectomy for Chronic Pelvic Pain. Obstet Gynecol 2016;127:1045-53. [Crossref] [PubMed]
- Lamvu G. Role of hysterectomy in the treatment of chronic pelvic pain. Obstet Gynecol 2011;117:1175-8. [Crossref] [PubMed]
- Brandsborg B, Nikolajsen L, Hansen CT, et al. Risk factors for chronic pain after hysterectomy: a nationwide questionnaire and database study. Anesthesiology 2007;106:1003-12. [Crossref] [PubMed]
- Hillis SD, Marchbanks PA, Peterson HB. The effectiveness of hysterectomy for chronic pelvic pain. Obstet Gynecol 1995;86:941-5. [Crossref] [PubMed]
- Hartmann KE, Ma C, Lamvu GM, et al. Quality of life and sexual function after hysterectomy in women with preoperative pain and depression. Obstet Gynecol 2004;104:701-9. [Crossref] [PubMed]
- Stovall TG, Ling FW, Crawford DA. Hysterectomy for chronic pelvic pain of presumed uterine etiology. Obstet Gynecol 1990;75:676-9. [PubMed]
- Farquhar CM, Harvey SA, Yu Y, et al. A prospective study of 3 years of outcomes after hysterectomy with and without oophorectomy. Am J Obstet Gynecol 2006;194:711-7. [Crossref] [PubMed]
- Magtibay PM, Magrina JF. Ovarian remnant syndrome. Clin Obstet Gynecol. 2006;49:526-34. [Crossref] [PubMed]
- Nezhat F, Nezhat C. Operative laparoscopy for the treatment of ovarian remnant syndrome. Fertil Steril 1992;57:1003-7. [Crossref] [PubMed]
- Magtibay PM, Nyholm JL, Hernandez JL, et al. Ovarian remnant syndrome. Am J Obstet Gynecol 2005;193:2062-6. [Crossref] [PubMed]
- Lemos N, Possover M. Laparoscopic approach to intrapelvic nerve entrapments. J Hip Preserv Surg 2015;2:92-8. [Crossref] [PubMed]
- Possover M, Andersson KE, Forman A. Neuropelveology: An Emerging Discipline for the Management of Chronic Pelvic Pain. Int Neurourol J 2017;21:243-6. [Crossref] [PubMed]
- Possover M. Use of the LION procedure on the sensitive branches of the lumbar plexus for the treatment of intractable postherniorrhaphy neuropathic inguinodynia. Hernia 2013;17:333-7. [Crossref] [PubMed]
- Kale A, Basol G, Usta T, et al. Laparoscopic evaluation of female pelvic neuroanatomy and autonomic plexuses in terms of gynecologic perspective. J Endometr Pelvic Pain Disord 2018;10:216-21. [Crossref]
- Doyle JB. Paracervical uterine denervation by transection of the cervical plexus for the relief of dysmenorrhea. Am J Obstet Gynecol 1955;70:1-16. [Crossref] [PubMed]
- Daniels JP, Middleton L, Xiong T, et al. Individual patient data meta-analysis of randomized evidence to assess the effectiveness of laparoscopic uterosacral nerve ablation in chronic pelvic pain. Hum Reprod Update 2010;16:568-76. [Crossref] [PubMed]
- Daniels J, Gray R, Hills RK, et al. Laparoscopic uterosacral nerve ablation for alleviating chronic pelvic pain: a randomized controlled trial. JAMA 2009;302:955-61. [Crossref] [PubMed]
- Johnson NP, Farquhar CM, Crossley S, et al. A double-blind randomised controlled trial of laparoscopic uterine nerve ablation for women with chronic pelvic pain. BJOG 2004;111:950-9. [Crossref] [PubMed]
- Chen FP, Chang SD, Chu KK, et al. Comparison of laparoscopic presacral neurectomy and laparoscopic uterine nerve ablation for primary dysmenorrhea. J Reprod Med 1996;41:463-6. [PubMed]
- Nezhat CH, Seidman DS, Nezhat FR, et al. Long-term outcome of laparoscopic presacral neurectomy for the treatment of central pelvic pain attributed to endometriosis. Obstet Gynecol 1998;91:701-4. [PubMed]
- Candiani GB, Fedele L, Vercellini P, et al. Presacral neurectomy for the treatment of pelvic pain associated with endometriosis: a controlled study. Am J Obstet Gynecol 1992;167:100-3. [Crossref] [PubMed]
- Zullo F, Palomba S, Zupi E, et al. Effectiveness of presacral neurectomy in women with severe dysmenorrhea caused by endometriosis who were treated with laparoscopic conservative surgery: a 1-year prospective randomized double-blind controlled trial. Am J Obstet Gynecol 2003;189:5-10. [Crossref] [PubMed]
- Hasson HM. Electrocoagulation of pelvic endometriotic lesions with laparoscopic control. Am J Obstet Gynecol 1979;135:115-21. [Crossref] [PubMed]
- Palomba S, Zupi E, Falbo A, et al. Presacral neurectomy for surgical management of pelvic pain associated with endometriosis: a descriptive review. J Minim Invasive Gynecol 2006;13:377-85. [Crossref] [PubMed]
- Chen FP, Soong YK. The efficacy and complications of laparoscopic presacral neurectomy in pelvic pain. Obstet Gynecol 1997;90:974-7. [Crossref] [PubMed]
- Latthe PM, Proctor ML, Farquhar CM, et al. Surgical interruption of pelvic nerve pathways in dysmenorrhea: a systematic review of effectiveness. Acta Obstet Gynecol Scand 2007;86:4-15. [Crossref] [PubMed]
- Possover M, Chiantera V, Baekelandt J. Anatomy of the Sacral Roots and the Pelvic Splanchnic Nerves in Women Using the LANN Technique. Surg Laparosc Endosc Percutan Tech 2007;17:508-10. [Crossref] [PubMed]
- Possover M, Schneider T, Henle KP. Laparoscopic therapy for endometriosis and vascular entrapment of sacral plexus. Fertil Steril 2011;95:756-8. [Crossref] [PubMed]
- Siquara De Sousa AC, Capek S, Amrami KK, et al. Neural involvement in endometriosis: Review of anatomic distribution and mechanisms. Clin Anat 2015;28:1029-38. [Crossref] [PubMed]
- Possover M, Baekelandt J, Flaskamp C, et al. Laparoscopic neurolysis of the sacral plexus and the sciatic nerve for extensive endometriosis of the pelvic wall. Minim Invasive Neurosurg 2007;50:33-6. [Crossref] [PubMed]
- Chiantera V, Petrillo M, Abesadze E, et al. Laparoscopic Neuronavigation for Deep Lateral Pelvic Endometriosis: Clinical and Surgical Implications. J Minim Invasive Gynecol 2018;25:1217-23. [Crossref] [PubMed]
- Possover M, Lemos N. Risks, symptoms, and management of pelvic nerve damage secondary to surgery for pelvic organ prolapse: a report of 95 cases. Int Urogynecol J 2011;22:1485-90. [Crossref] [PubMed]
- Possover M. Laparoscopic management of neural pelvic pain in women secondary to pelvic surgery. Fertil Steril 2009;91:2720-5. [Crossref] [PubMed]
- Lemos N, Marques RM, Kamergorodsky G, et al. Vascular entrapment of the sciatic plexus causing catamenial sciatica and urinary symptoms. Int Urogynecol J 2016;27:317-9. [Crossref] [PubMed]
- Possover M, Forman A. Pelvic Neuralgias by Neuro-Vascular Entrapment: Anatomical Findings in a Series of 97 Consecutive Patients Treated by Laparoscopic Nerve Decompression. Pain Physician 2015;18:E1139-43. [PubMed]
- Kale A, Basol G, Usta T, et al. Vascular Entrapment of Both the Sciatic and Pudendal Nerves Causing Persistent Sciatica and Pudendal Neuralgia. J Minim Invasive Gynecol 2019;26:360-1. [Crossref] [PubMed]
- Maleux G, Stockx L, Wilms G, et al. Ovarian vein embolization for the treatment of pelvic congestion syndrome: long-term technical and clinical results. J Vasc Interv Radiol 2000;11:859-64. [Crossref] [PubMed]
- Liddle AD, Davies AH. Pelvic congestion syndrome: chronic pelvic pain caused by ovarian and internal iliac varices. Phlebology 2007;22:100-4. [Crossref] [PubMed]
- Beard RW, Kennedy RG, Gangar KF, et al. Bilateral oophorectomy and hysterectomy in the treatment of intractable pelvic pain associated with pelvic congestion. Br J Obstet Gynaecol 1991;98:988-92. [Crossref] [PubMed]
- Soysal ME, Soysal S, Vıcdan K, et al. A randomized controlled trial of goserelin and medroxyprogesterone acetate in the treatment of pelvic congestion. Hum Reprod 2001;16:931-9. [Crossref] [PubMed]
- Chung MH, Huh CY. Comparison of treatments for pelvic congestion syndrome. Tohoku J Exp Med 2003;201:131-8. [Crossref] [PubMed]
Cite this article as: Yilmaz S, Topbas Selcuki NF, Usta T, Kale A. Minimally invasive surgery in pelvic pain: from a gynecological perspective. Gynecol Pelvic Med 2021;4:5.