
Evidence that cosmetic talc is a cause of ovarian cancer
O’Brien et al. (2020) published a meta-analysis of four cohorts of 252,745 women and concluded “there was not a statistically significant association between use of powder in the genital area and incident ovarian cancer (1).” We have published on the many erroneous assumptions that plagued talc-ovarian cancer cohort studies; however, these problems persisted in O’Brien et al. (2).
- Inadequate understanding of the asbestos content of talc powders.
- Similarities between ovarian cancer and asbestos-caused mesothelioma.
- Asbestos has been found in ovarian tumor tissue of talc users.
- Misclassification issues in ovarian cancer-talc epidemiology studies:
- Inadequate characterization of exposure.
- Conflating Cornstarch and Talc.
- Misclassification of perineal exposures.
- Inadequate follow-up and latency.
- Recall Bias as an explanation for the different findings in cohort and case-control studies.
- “Nonsignificant” conclusion.
- Applying Hill’s Considerations to the Question of the Relationship between Talc Use and Ovarian Cancer.
Contrary to O’Brien et al., neither the U.S government nor the talc industry ever banned the presence of asbestos in “cosmetic” talc (2). In fact, since the 1950s and as recently as October 2019, talc manufacturing companies and the FDA have found asbestos in cosmetic talc products and ores (2). During perineal and other body applications of cosmetic talc, users inhale talc and asbestos (3,4). Inhaled asbestos transmigrates through the lymphatic system to the peritoneum ovary and adjacent tissues (5,6). Exposures during cosmetic talc use are high enough to cause talcosis in some users (7).
Inhaled asbestos is an established cause of mesothelioma, ovarian and lung cancers (2,8). Peritoneal mesotheliomas and serous ovarian cancer are histologically similar and often difficult to distinguish (9-11). The peritoneum, pleura, ovary and fallopian tubes all originate in the mesoderm and their tumors are “histologically and clinically” similar (2,12,13). Mesothelioma and serous ovarian cancer frequently exhibit p53 chromosomal deletions (11). Asbestos has been shown to induce p53 deletions in vitro (14). Gordon et al. (2019) found that cosmetic talc contained asbestos and was “a causative agent in the development of mesotheliomas, lung tumors, gastrointestinal tumors, and ovarian tumors (7).”
Steffen et al. (2020) reported tissue analysis of ovarian tumors removed from ten talc users: asbestos was detected in in tissue samples from eight cases and fibrous talc was detected in all ten cases (4). Similarly, Emory et al. (2020) found asbestos in the lymph nodes and ovary of a cosmetic talc user who only had inhalation exposure (15,16). The specific combination of asbestos fiber types, tremolite and anthophyllite, found in tumor tissue is a “fingerprint” that is unique to fiber types present in “cosmetic” talc ores and products (4). Findings “fingerprints” of asbestos found in talc in ovarian cancer tissue is evidence that asbestos exposures from cosmetic talc use are sufficient to cause ovarian cancer.
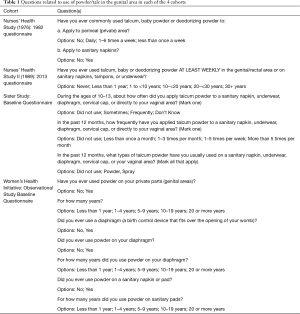
O’Brien et al. only considered perineal exposure in adults (1,5) (Table 1). However, most female inhalation exposures occur during diapering and adult upper body use (4). Johnson & Johnson (J&J) estimated that over 130 million babies born prior to 1992 were diapered with talc and “75% of teen girls and 80% of women use a talc (2,17).” O’Brien et al. ignore many other uses that result in inhalation exposure including application to the upper body, sheets and pillows; dry shampoo; and pet flea powders. For example, Glickman et al. attributed a statistically significant 11-fold increase in mesothelioma in dogs whose owners used talc flea powders (18).” By focusing exclusively on perineal exposure, O’Brien et al. randomly misclassified and underestimated talc and asbestos exposure.
O’Brien et al. (2020) claimed “to evaluate the talc-ovarian cancer association using prospective data (1).” However, three of the four included cohort studies conflated cornstarch and talc powder use (2). For example, the Women’s Health Initiative only asked “Have you ever used powder on your private parts (genital areas)?” without specifying the type of powder that was used (Table 1). Thus, exposures in O’Brien et al. (2020) are seriously misclassified in a way that diminishes associations (2). Such misclassification—the inclusion of un-exposed cases in the exposure group—introduces bias towards the null.
O’Brien et al. (2020) claimed to compare “Ever, long-term (20 years), and frequent (1/week) use of powder in the genital area.” However, neither the Nurses’ Health Studies nor O’Brien 2013 questionnaires asked subjects if they ever used talc (1). For example, the Nurses’ Health Study asked their participants “Have you ever commonly used talcum, baby powder or deodorizing powder.” (See Table 1) Furthermore, O’Brien et al. (2020)’s criteria for “ever,” “frequent” and “long term” talc use are inconsistent. (See Table 2) In addition, the underlying studies only evaluated talc exposure at a single point in time. It is likely that talc use changed after it was assessed, as some use was associated with application during menstruation (2).
The Nurses’ Health Study II began in 1989 but did not collect information on talc use (1). In 2013, O’Brien created a retrospective questionnaire about talc use for the Nurses’ Health Study II (1). As a result, O’Brien only analyzed 76 ovarian cases that occurred after the 2013 questionnaire. O’Brien et al. (2020)’s analysis of the “updated” Nurses’ Health Study II only had, on average, 3.8 years follow-up time even though pre-2013 talc use would have influenced the result if talc exposure caused ovarian cancer (1). For example, O’Brien’s analysis omits all of the 287 patients who may have used talc between 1989 and 2013 but were diagnosed with ovarian cancer before the 2013 questionnaire. O’Brien et al. (2020)’s overall average follow-up time is 11.2 (3.9–21.0) years (1). The latency for ovarian cancer is from 25 years to “several decades (19,20).”
O’Brien et al. argued that previous case-control results “may be affected by recall bias.” However, recall bias cannot explain the inconsistent association between talc use and different ovarian cancer sub-types. Berge et al. (2018), a meta-analysis of case-control and cohort studies, found that the association between talc use and ovarian cancer varied by histologic type, with no evidence of association for mucinous and clear cell carcinomas (21).” Similarly, Penninkilampi et al. (2018) reported that an increased risk of serous and endometrioid, but not mucinous or clear cell subtypes (22).” Recall bias, if it existed, would have operated across all histologic types (21).
O’Brien et al. observed a “nonsignificant” risk increase of (HR, 1.08; 95% CI, 0.99–1.17). This does not represent affirmative evidence that talc is not a cause of ovarian cancer. In fact, the study fails to reject the null hypothesis that there is a 17% increased risk. Furthermore, under any reasonable prior, the posterior probability that that true risk exceeds 1 is greater than 95%. This “nonsignificance” reflects the study’s low power for detecting risk increases, and is unsurprising, as power depends on the number of ovarian cancer cases, not the number of patients enrolled. O’Brien et al. reported 2,168 ovarian cancer cases while Taher et al. (2019) reported 15,063 ovarian cancer cases from case-control studies (23). Therefore, the number of enrolled patients (252,745 women) does not per se indicate that the cohort meta-analysis was more powered to find a statistical significant result than that found in the case-control studies which have more ovarian cancer cases. Etikan et al. (2016) stated “Computationally, both approaches lead to the same result but the case control study has a greater statistical power than cohort studies, which must often wait for a ‘sufficient’ number of disease events to accumulate (24).”
Non-statistically significant results do not establish an absence of risk. A meta-analysis of epidemiological case-control studies reported a relative risk of 1.35 (95% CI, 1.27–1.43) for talc usage and ovarian cancer (22). A meta-analysis of cohort studies found a statistically significant association between talc use and invasive serous type ovarian cancer (OR, 1.25; 95% CI, 1.01–1.55) (22).
In 1965, to rebut tobacco company arguments on the safety of cigarette smoke, Sir Bradford Hill published a set of considerations to be used to access the carcinogenicity of an environmental or occupational exposure (25). These considerations are: strength of association, specificity, temporality, consistency, biological gradient, plausibility, coherence, experimental evidence, and analogy (25).
We apply these considerations to evaluate the question, “Does asbestos and fibrous talc as found in cosmetic talc contribute to the development of ovarian cancer?”
Full table

Full table
Strength of association
IARC 100c “examined 11 cohort studies that examined the association between asbestos exposure and ovarian cancer in 13 populations, ten with occupational exposure to asbestos and three with community-based or residential exposure (26).” For each of these studies the Standardized Mortality Ratio (SMR) ranged from 1.26 to 5.35. IARC determined a causal link between inhalation of asbestos and ovarian cancer (26). This causal link in turn applies to cosmetic talc which contains asbestos.
A meta-analysis by Camargo et al. (2011) reported a pooled rate of 1.77 (95% CI, 1.37–2.28) based on 20 occupational cohort studies (27).” Camargo et al. reaffirmed that “Our study supports the IARC conclusion that exposure to asbestos is causally associated with increased risk of ovarian cancer.” Penninkilampi et al. (2018) conducted a meta-analysis of 31 ovarian cancer epidemiologic studies that examined the relationship between perineal talc application and ovarian cancer (22). Researchers in the underlying studies Penninkilampi et al. (2018) reviewed did not consider inhalation exposure or exposure during diapering and thus suffered from systematic misclassification of exposed and control cases and underestimations of exposure both of which bias the results toward the null (2). Nonetheless Penninkilampi et al. (2018) found that any perineal talc use was associated with increased risk of ovarian cancer (OR, 1.31; 95% CI, 1.24–1.39). An association with ever use of talc was found in case-control studies (OR, 1.35; 95% CI, 1.27–1.43), but not cohort studies (OR, 1.06; 95% CI, 0.90–1.25).” Penninkilampi et al. (2018) evaluation of cohort studies, “found an association between talc use and invasive serous type ovarian cancer (OR, 1.25; 95% CI, 1.01–1.55). Penninkilampi et al. (2018) concluded, “there is a consistent association between perineal talc use and ovarian cancer (22).” Overall the studies show a 30% increase in cases with exposure. This is in the same range of various risk factors that are accepted causes of cardiovascular disease (28).
Consistency
Studies demonstrate consistency across subjects, location, circumstance and time. Camargo et al. (2011) reported only three results included in their meta-analysis had an SMR point estimate less than one (27). Camargo and IARC respectively found that 85% and 93% of studies analyzed revealed an increased risk of ovarian cancer with asbestos exposure (27). Camargo et al. reported studies of populations in the UK, Australia, Italy, Germany, Denmark, Finland, Iceland, Norway and Sweden that have found an elevated risk of ovarian cancer in women exposed to asbestos (27). IARC identified 14 studies on asbestos and ovarian cancer and only one (Reid et al. 2009) had an SMR below one (29).
Specificity
Specificity is not reliable as a causal factor since many carcinogens cause multiple cancers and non-malignant diseases (26). For example, smoking causes cancer of the lung, larynx, esophagus, mouth, lip, and stomach, as well as heart disease, strokes, Buerger’s disease, ulcers and other diseases (30). In this case, inhaled asbestos causes mesothelioma, ovarian cancer, and other types of cancer.
Temporality
Temporality is met in all studies because exposure to talc always preceded the outcome—ovarian cancer (27).
Biologic gradient (dose-response)
Wignall et al. found that the “some exposure” group of talc users had a SMR of 2.78 while the “heavy exposure” group had a SMR of 14.81 for asbestos and ovarian cancer (31). Berry found an increasing non-statistically significant increase in ovarian cancer rates comparing “low/moderate” exposure to “severe” exposure (>2 years) (32). Penninkilampi et al. (2018) conducted a meta-analysis of 31 ovarian cancer epidemiologic studies that examined the relationship between perineal talc application and ovarian cancer (22). They found a dose response relationship and elevated risk, “Higher use (> than 3,600 lifetime applications had an OR, 1.42; 95% CI, 1.25–1.61 were slightly more associated with ovarian cancer than <3,600 (OR, 1.32; 95% CI, 1.15–1.50).”
Biologic plausibility
It is well established that inhaled asbestos is the main cause of peritoneal mesothelioma in asbestos exposed populations (33-36). Several studies have demonstrated the ability of asbestos and talc to migrate to the ovaries after inhalation. In 1971, Henderson et al. reported the incidence of talc and asbestos in a case series of patients with cervical or ovarian cancer (37). They found talc particles in 10/13 ovarian tumors and 12/21 cervical tumors (37). J&J obtained the ovarian tissue from Henderson and sent it to Dr. Langer at Mount Sinai for evaluation. Langer found asbestos and talc in Henderson’s ovarian cancer tissue (38). Henderson et al. demonstrated that talc migrated to the ovaries from the vagina (39). Heller et al. found “significant asbestos fiber burdens” in the ovaries of 9 out of 13 women with household asbestos exposure (6,40,41). Other studies by Werebe et al. and Cramer et al. confirm the finding of talc and asbestos in ovarian tissue (26,42,43). As noted above Steffen et al. (2020) reported tissue analysis of ovarian tumors removed from ten talc users and found tremolite and/or anthophyllite in their ovarian tissue samples in addition to talc (4). Talc was present in tumor tissue in all cases. Talc is the only commercial product that contains both tremolite and anthophyllite. The singular presence of these two asbestos fiber types is a finger print for talc exposure. These cases provide more evidence of the causal link between asbestos, talc, and ovarian cancer and indicate that asbestos is present in consumer talc products at a level sufficient to cause disease.
Serous ovarian cancer and mesothelioma are histologically and clinically similar (44). Mutations are a cause of ovarian cancer, and asbestos induces P53 mutations and 80% of serous ovarian cancers have P53 mutations (14,45-48). The ovary and peritoneum have the same embryologic mesodermal origin (44,49).
Coherence
The data is coherent based on consistency, biologic plausibility and strength of association. The data is not inconsistent with any known biologic models or theories.
Analogy
There are no other known fibers that have been evaluated as possible causes of ovarian cancer. A case-control study of mesothelioma in domestic dogs concluded that there was an association between the incidence of mesothelioma and asbestos exposure; the source of exposure of the dogs was from the use of talc containing flea powders and/or the owner's asbestos-related occupations (hobbies) (18).
Experiment
In 1967, Graham and Graham published their series of experiments on asbestos and ovarian cancer in animals (50). Graham and Graham injected tremolite into the peritoneum of mice, hamsters, guinea pigs, and rabbits over 18 weeks. Animals were killed in 1-4-week intervals and ovaries were examined upon autopsy. Graham and Graham observed surface abnormalities “reminiscent of changes seen in early ovarian lesions in humans” on the ovaries of 2 of 10 exposed rabbits and 2 of 16 exposed guinea pigs; no ovarian abnormalities were observed among the controls.
There are no other experimental studies directly evaluating the risk of ovarian cancer in animals exposed to asbestos. However, several studies have evaluated the incidence of ovarian cancer in animals exposed to talc, which likely contained asbestos as an accessory mineral. These did not find an increase in ovarian cancer (51,52). Wehner et al. (1977) lacked sufficient latency (53).
Conclusions
Based on Hill’s considerations, there is sufficient evidence that cosmetic talc products cause or contribute to the development of serous ovarian cancer.
In 1974, J&J told the FDA that, “…if the results of any scientific studies show any question of safety of talc, Johnson & Johnson will not hesitate to take it off the market (2).” Studies like O’Brien et al., which are based on post-hoc hypotheses and mining of flawed data, fail to answer the question of the safety of talc. For those readers who (erroneously) believe statistical significance is a key measure of effect, one analysis in the study supports the inference that talc exposure increases the risk of ovarian cancer. O’Brien et al. (2020) reported a statistically significant ovarian cancer risk increase in women who used cosmetic talc with no history of tubal ligation (HR, 1.10; 95% CI, 1.00–1.21) (1). Thus “cosmetic” talc should be avoided, for it has no medical benefit and cornstarch is a safe substitute. We agree with J&J: cosmetic talc should not be sold. On May 19, 2020, J&J stopped selling talc containing baby powder in the United States and Canada.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Gynecology and Pelvic Medicine. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-28/coif). DE reports personal fees from Kazan Law Firm, personal fees from Lanier Law firm, outside the submitted work. DM serves as an expert witness in litigation at the request of people who claim injuries resulting from the use of talcum powders. He was not compensated for work on this commentary. No party to these litigations reviewed this commentary or had input into its content. MY is an employee of DE. TT is an employee of DE.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- O'Brien KM, Tworoger SS, Harris HR, et al. Association of Powder Use in the Genital Area With Risk of Ovarian Cancer. JAMA 2020;323:49-59. [Crossref] [PubMed]
- Tran TH, Steffen JE, Clancy KM, et al. Talc, Asbestos, and Epidemiology: Corporate Influence and Scientific Incognizance. Epidemiology 2019;30:783-8. [Crossref] [PubMed]
- Moline J, Bevilacqua K, Alexandri M, et al. Mesothelioma Associated With the Use of Cosmetic Talc. J Occup Environ Med 2020;62:11-7. [Crossref] [PubMed]
- Steffen JE, Tran T, Yimam M, et al. Serous Ovarian Cancer Caused by Exposure to Asbestos and Fibrous Talc in Cosmetic Talc Powders-A Case Series. J Occup Environ Med 2020;62:e65-77. [Crossref] [PubMed]
- Miserocchi G, Sancini G, Mantegazza F, et al. Translocation pathways for inhaled asbestos fibers. Environ Health 2008;7:4. [Crossref] [PubMed]
- Heller DS, Gordon RE, Westhoff C, et al. Asbestos exposure and ovarian fiber burden. Am J Ind Med 1996;29:435-9. [Crossref] [PubMed]
- Gordon RE. Cosmetic Talcum Powder as a Causative Factor in the Development of Diseases of the Pleura. In: Stojšić J. editor. Diseases of Pleura. IntechOpen, 2019.
- International Agency for Research on Cancer (IARC). IARC Monographs On The Evaluation Of Carcinogenic Risks To Humans. Volume 78 Ionizing Radiation, Part 2: Some Internally Deposited Radionuclides. 2001.
- Inai K. Pathology of mesothelioma. Environ Health Prev Med 2008;13:60-4. [Crossref] [PubMed]
- Ordóñez NG. Value of estrogen and progesterone receptor immunostaining in distinguishing between peritoneal mesotheliomas and serous carcinomas. Hum Pathol 2005;36:1163-7. [Crossref] [PubMed]
- Gates MA, Tworoger SS, Terry KL, et al. Talc use, variants of the GSTM1, GSTT1, and NAT2 genes, and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev 2008;17:2436-44. [Crossref] [PubMed]
- Ferretti A. A Review of the Overlapping Genetic Mechanisms Between Ovarian Carcinomas and Malignant Mesothelioma. Presentation at the American Public Health Association Annual Meeting and Expo, Philadelphia, 2019.
- Kurman RJ, Shih IeM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 2010;34:433-43. [Crossref] [PubMed]
- Appel JD, Fasy TM, Kohtz DS, et al. Asbestos fibers mediate transformation of monkey cells by exogenous plasmid DNA. Proc Natl Acad Sci U S A 1988;85:7670-4. [Crossref] [PubMed]
- Emory TS, Maddox JC, Kradin RL. Malignant mesothelioma following repeated exposures to cosmetic talc: A case series of 75 patients. Am J Ind Med 2020;63:484-9. [Crossref] [PubMed]
- Emory T. to Egilman D. Personal Communication. Question on Case 75. April 2020. Available online: https://repository.library.brown.edu/studio/item/bdr:1111086/
- Wells P. Memo to Mr. H. R. Callum Re: Johnson's Baby Powder Review of Consumer Research. 1973. Available online: https://repository.library.brown.edu/studio/item/bdr:1111087/
- Glickman LT, Domanski LM, Maguire TG, et al. Mesothelioma in pet dogs associated with exposure of their owners to asbestos. Environ Res 1983;32:305-13. [Crossref] [PubMed]
- Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst 1998;90:1774-86. [Crossref] [PubMed]
- Gosvig CF, Kjaer SK, Blaakær J, et al. Coffee, tea, and caffeine consumption and risk of epithelial ovarian cancer and borderline ovarian tumors: Results from a Danish case-control study. Acta Oncol 2015;54:1144-51. [Crossref] [PubMed]
- Berge W, Mundt K, Luu H, et al. Genital use of talc and risk of ovarian cancer: a meta-analysis. Eur J Cancer Prev 2018;27:248-57. [Crossref] [PubMed]
- Penninkilampi R, Eslick GD. Perineal Talc Use and Ovarian Cancer: A Systematic Review and Meta-Analysis. Epidemiology 2018;29:41-9. [Crossref] [PubMed]
- Kadry Taher M, Farhat N, Karyakina NA, et al. Critical review of the association between perineal use of talc powder and risk of ovarian cancer. Reprod Toxicol 2019;90:88-101. [Crossref] [PubMed]
- Etikan I, Alkassim R, Abubakar S. Meta-analysis of using both cohort and case control study. Biom Biostat Int J 2016;3:68. [Crossref]
- Hill AB. The Environment and Disease: Association or Causation? Proc R Soc Med 1965;58:295-300. [Crossref] [PubMed]
- International Agency for Research on Cancer (IARC). Arsenic, Metals, Fibres, and Dusts. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2012;100C.
- Camargo MC, Stayner LT, Straif K, et al. Occupational Exposure to Asbestos and Ovarian Cancer: A Meta-analysis. Environmental Health Perspectives 2011;119:1211-7. [Crossref] [PubMed]
- Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016;133:e38-360. [PubMed]
- Reid A, Segal A, Heyworth JS, et al. Gynecologic and breast cancers in women after exposure to blue asbestos at Wittenoom. Cancer Epidemiol Biomarkers Prev 2009;18:140-7. [Crossref] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum 2004;83:1-1438. [PubMed]
- Wignall BK, Fox AJ. Mortality of female gas mask assemblers. Br J Ind Med 1982;39:34-8. [PubMed]
- Berry G, Newhouse ML, Wagner JC. Mortality from all cancers of asbestos factory workers in east London 1933-80. Occup Environ Med 2000;57:782-5. [Crossref] [PubMed]
- Selikoff I, Churg J. Biological Effects of Asbestos. Ann N Y Acad Sci 1965;132:766.
- Newhouse ML. Epidemiology of Mesothelial Tumors in the London Area. Ann N Y Acad Sci 1965;132:579-88. [Crossref] [PubMed]
- Wagner JC. Asbestos Dust Deposition and Retention in Rats. Ann N Y Acad Sci 1965;132:77-86. [Crossref] [PubMed]
- Wagner JC. The Sequelae of Exposure to Asbestos Dust. Ann N Y Acad Sci 1965;132:691-5. [Crossref] [PubMed]
- Henderson WJ, Joslin CA, Turnbull AC, et al. Talc and carcinoma of the ovary and cervix. J Obstet Gynaecol Br Commonw 1971;78:266-72. [Crossref] [PubMed]
- Langer AM. Letter to Henderson. Re: Testing Results. 1971. Available online: https://repository.library.brown.edu/studio/item/bdr:858491/
- Henderson WJ, Hamilton TC, Baylis MS, et al. The demonstration of the migration of talc from the vagina and posterior uterus to the ovary in the rat. Environ Res 1986;40:247-50. [Crossref] [PubMed]
- Heller DS, Gordon RE, Katz N. Correlation of asbestos fiber burdens in fallopian tubes and ovarian tissue. Am J Obstet Gynecol 1999;181:346-7. [Crossref] [PubMed]
- Heller DS, Westhoff C, Gordon RE, et al. The relationship between perineal cosmetic talc usage and ovarian talc particle burden. Am J Obstet Gynecol 1996;174:1507-10. [Crossref] [PubMed]
- Werebe EC, Pazetti R, Milanez de Campos JR, et al. Systemic distribution of talc after intrapleural administration in rats. Chest 1999;115:190-3. [Crossref] [PubMed]
- Cramer DW, Welch WR, Berkowitz RS, et al. Presence of talc in pelvic lymph nodes of a woman with ovarian cancer and long-term genital exposure to cosmetic talc. Obstet Gynecol 2007;110:498-501. [Crossref] [PubMed]
- Parmley TH. The ovarian mesothelioma. American Journal of Obstetrics and Gynecology 1974;120:234-41. [Crossref] [PubMed]
- Herrington CS, McCluggage WG. The emerging role of the distal Fallopian tube and p53 in pelvic serous carcinogenesis. J Pathol 2010;220:5-6. [Crossref] [PubMed]
- Iwanicki MP, Chen HY, Iavarone C, et al. Mutant p53 regulates ovarian cancer transformed phenotypes through autocrine matrix deposition. JCI Insight 2016;1:e86829. [Crossref] [PubMed]
- Meinhold-Heerlein I, Fotopoulou C, Harter P, et al. The new WHO classification of ovarian, fallopian tube, and primary peritoneal cancer and its clinical implications. Arch Gynecol Obstet 2016;293:695-700. [Crossref] [PubMed]
- Di Marzo D. Pharmacological targeting of p53 through RITA is an effective antitumoral strategy for malignant pleural mesothelioma. Cell Cycle 2014;13:652-65. [Crossref] [PubMed]
- Hancock KL, Clinton CM, Dinkelspiel HE, et al. A case of mesothelioma masquerading pre-operatively as ovarian cancer and brief review of the literature. Gynecol Oncol Rep 2016;17:26-8. [Crossref] [PubMed]
- Graham J, Graham R. Ovarian cancer and asbestos. Environ Res 1967;1:115-28. [Crossref] [PubMed]
- National Toxicology Program (NTP). Toxicology and Carcinogenesis Studies of Talc in F344/N Rats and B6C3F1 Mice. Available online: https://repository.library.brown.edu/studio/item/bdr:858543/
- Wehner AP, Zwicker GM, Cannon WC. Inhalation of talc baby powder by hamsters. Food Cosmet Toxicol 1977;15:121-9. [Crossref] [PubMed]
- Johnson & Johnson (J&J). Report on Meeting with Dr Francis Roe on Monday, November 13th 1972. 1972.11.13 how to create a negative animal cancer study Tobacco, JNJTALC000290381. Available online: https://repository.library.brown.edu/studio/item/bdr:1111088/
Cite this article as: Egilman D, Madigan D, Yimam M, Tran T. Evidence that cosmetic talc is a cause of ovarian cancer. Gynecol Pelvic Med 2021;4:2.


