Robotic fistula repair of multiple vesicovaginal fistulas using sigmoid epiploica: case report
Introduction
Urogenital fistulas are among the most common gynecologic complication worldwide, especially in low-resourced countries. In women with pelvic surgery, the overall rate of urinary tract injury ranges from 0.3 to nearly 1 percent (1). Bladder injury is as much as three times more common than ureteral injury (2). They are significantly less common in the United States, with the vast majority of fistulas resulting from surgery, particularly hysterectomies (3). Despite their relatively low incidence, vesicovaginal fistulas (VVFs) are the most common type of urinary tract fistulas in well-resourced countries (4). Fistulas are a source of emotional and psychological strain on affected patients (5), so it is important to recognize and treat this complication quickly. Symptoms of a VVF generally occur within two weeks of surgery and include continuous urinary leakage, often described as a thin vaginal discharge. The diagnosis of the vesicovaginal fistula may be made on a physical exam with visual confirmation of urine in the vaginal vault and with cystoscopy.
Surgical repair options include trans-abdominal (open, laparoscopic, and robotic-assisted) and vaginal approaches. There is no randomized clinical trial that compares the four approaches to determine superiority (6). However, each approach has advantages and disadvantages and have benefits in certain clinical scenarios. Vaginal approaches have the advantage of being minimally invasive (4) and having shorter operative times (5).
However, vaginal approaches may not be technically possible if there is pre-existing vaginal shortening (4,5), the fistula is too high for vaginal access, or there is pre-existing vaginal scarring (4,7). The first reported case of robotic-assisted laparoscopic VVF repair was in 2005 (8). This approach is advantageous because there is generally a very low EBL, with a case series of 10 robotic-assisted VVF repairs showing a mean EBL of 43 mL (4). Further, robotic-assisted laparoscopic repair provides a three-dimensional view of pelvic anatomy that allows for superior identification of tissue planes, accurate dissection between the bladder and vagina, and preservation of vascular supply of bladder and vaginal flaps (4). Robotic-assisted laparoscopic repair also provides for rapid recovery enhanced adhesiolysis, shorter hospital stays compared to open repair (5), and elimination of operator tremor compared to open and laparoscopic repair (9). A difficulty with any abdominal repair procedure is the identification of the fistula tract.
Within the trans-abdominal approaches are two intraoperative techniques for fistula repair: transvesical (O’Conor Technique) and extravesical techniques. The transvesical technique requires a 4–5 cm cystotomy intentionally cut near the bladder dome, with dissection within the vesicovaginal plane at least 1–2 cm distal to the fistula. This technique is the gold standard for supratrigonal VVF (5). This technique allows for the exact localization of the fistula (4,6) and potential debridement of nearby unhealthy tissue (4). However, this technique is more invasive than the extravesical technique and requires catheterization for a more extended period of time (7). The extravesical technique avoids cystotomy by threading a stent from the vagina to the bladder via the fistulous tract, allowing for visualization of the stent. This is less invasive and less traumatic to the healthy bladder tissue (4,6). However, this technique may not be possible if the surgeon is unable to locate the fistulous tract (6) or the fistula is too small for the stent to pass through (7). Alternatively, the assistant can locate the VVF with a cystoscope and use the light of the cystoscope to focus on the fistula. This light can then be identified laparoscopically, thus identifying the location of the fistula without the need for cystotomy (10). No study has definitively found one approach to be more successful than the other. A systematic review of 44 articles and 256 total patients comparing transvesical and extravesical techniques found no statistical significance between success rates, defined as no urinary leakage from the vagina at post-operative follow-up appointment (96% vs. 98%, RR 0.98, CI, 0.94–1.02) (11). Overall, the approach that will be the most successful is the one that is chosen based on the surgeon’s experience and the fistula’s characteristics (5).
If the VVF is small and identified immediately post-operatively, it can be managed conservatively with a Foley catheter and anticholinergic treatment for 2–8 weeks. This approach successfully closes fistulous tracts in approximately 10% of cases (5). However, if the VVF is diagnosed several weeks post-operatively, surgery is recommended, with the approach dependent on the cause, location, and size of the fistula and the surgeon’s experience (4). After identification of the fistula, it can be repaired immediately, or repair can be delayed for 6–12 weeks to allow for resolution of granulation tissue (5). Most surgeons do not administer additional antibiotic prophylaxis when a urinary tract injury occurs, whether it is recognized intra- or postoperatively.
For the best chance at successful closure of a VVF there should be adequate fistulous tract exposure, favorable repair tissue conditions (good vascularity, no infection/inflammation), complete excision of fistulous tract, tension-free and watertight anastomosis, multilayered closure with non-overlapping suture lines, interposition of vascularized tissue between suture lines, and continuous post-operative bladder drainage until repair adequately healed (4). Several techniques requiring the use of grafted tissue have been described to assist with non-overlapping suture lines. The most common technique uses an omental flap to separate the suture lines. The omental flap is harvested by mobilizing a section—usually, one supplied by right gastroepiploic vessels—and suturing it to the anterior vaginal wall or posterior bladder wall. The theoretical advantages of this technique include the introduction of vascular and lymphatic supply to improve tissue growth (4,7). The disadvantage of utilizing this technique is that it increases the operative time (5) and can often be difficult to achieve in patients with extensive adhesions from prior surgeries (4). While the benefits of using an omental flap seem logical, studies have shown that there is no significant improved cure rate in procedures utilizing an omental graft and those utilizing no graft, with 96.3% and 98% success, respectively (11). In fact, the authors of the Miklos, Moore, and Chinthakanan study found that upon reoperation and vesicovaginal junction dissection, there was no increased vascularity or even the presence of the graft itself (11).
An alternative method, similar to the one utilized in the case of our patient, involves using the sigmoid colonic epiploica. It is preferred in cases where there are extensive omental adhesions preventing the use of omentum (4). It also involves a relatively shorter operative time than the use of omental flap. Again, the goal of using this tissue is to introduce vascular and lymphatic vessels to improve growth and repair (4,5,7) and potentially decrease the risk of infection and fluid collection (5). However, these theoretical benefits remain unproven in current literature (11).
We present the following case in accordance with the CARE Guidelines.
Case report
The patient is a 39-year-old G2P2 female who presented to the clinic with new-onset urinary leakage for three months, following total laparoscopic hysterectomy and bilateral salpingo-oophorectomy for endometriosis. The prior surgery was complicated by cystotomy for which she was discharged with a foley catheter for 2 weeks post-operative. After removal of the catheter, the patient had constant urinary leakage unrelated to Valsalva or urge, requiring her to wear a diaper. Physical exam revealed pooling of fluid in the vagina consistent with urine. The retrograde cystourethrogram confirmed a vesicovaginal fistula.
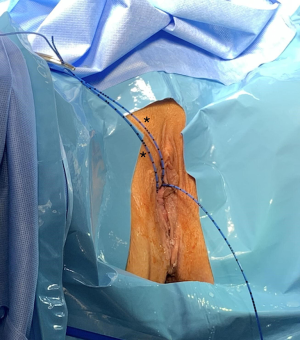
A decision was made to proceed with robotic-assisted vesicovaginal fistula repair to allow improved visualization of the fistula. Prophylactic antibiotics were administered, and the patient was positioned in dorsal lithotomy. Prior to robotic docking, a vaginoscopy and cystoscopy were performed for the identification of the ureters and fistula. Cystoscopy revealed two vesicovaginal fistulas, 1 cm apart from one another near the bladder dome and close in proximity to the right ureter. Using a rigid cystoscope with a 30-degree lens, two curve tip guide wires were placed into the fistula, followed by a 6F ureteral stent over the guidewire (Figure 1). These stents are seen exiting out of the fistula into the vagina. An additional catheter was placed in the right ureter.
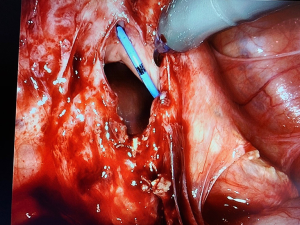
The abdomen was entered and insufflated using a Veress needle. Significant omental adhesions to the anterior abdominal wall were noted, and we proceeded with laparoscopic lysis of adhesions prior to robotic docking. Four robotic trocars were placed, and the patient was placed in slight trendelenburg. An EEA sizer was placed to manipulate the vaginal vault. The vesicovaginal space was entered, and dissection was performed. During dissection a cystostomy was created and the two fistulas were identified. The location of the right ureter was also identified due to the previously placed stent. We proceeded with cold excision around the fistula, away from ureteral openings to excise the fistulous tract. The cystostomy was closed in three layers using 2-0 vicryl in a continuous stitch in a tension-free manner. The bladder was back-filled with 300 mL normal saline mixed with one vial of methylene blue, which confirmed adequate closure. The corresponding vaginotomy (Figure 2) was repaired with a V-Loc running suture. Due to omental adhesions, a flap was created with the colonic epiploica from the nearby sigmoid colon, secured using a V-Loc suture. Cystoscopy was repeated, confirming complete vesicovaginal fistula closure, and ureteral stent was removed without difficulty. EBL was 20 mL. A Foley catheter was inserted for 7 days. The patient was discharged home with prophylactic antibiotics. One week post-operatively, retrograde cystourethrogram revealed no fistula or extravasation of contrast and the Foley catheter was removed.
Discussion
In our case, the patient developed a VVF following hysterectomy, similar to what the literature has reported being the most common etiology among highly resourced countries. Our patient also had a prior diagnosis of endometriosis, a condition noted to distort pelvic anatomy, making it more difficult to identify surgical dissection planes correctly (9). We describe our experience with using ureteral stents to assist in the identification of not only the ureters but also the fistulas. We also describe a unique approach using the sigmoid epiploica as an alternative to the omental flap. We also performed the transvesical technique for fistula repair given that the fistula was supratrigonal.
However, what makes this fistula repair unique was the use of the sigmoid epiploica in order to separate the suture lines between the bladder and vagina. In our search for articles published in the last five years describing the repair of VVF, only two studies cite the use of the sigmoid epiploica. Most studies cite an omental flap as the most common method for separating bladder and vaginal suture lines. However, our patient had significant omental adhesions as a result of prior abdominal surgeries, preventing the use of omentum. While Miklos, Moore, and Chinthakanan cited no difference in VVF cure rates between those with and without intervening tissue, a decision was made to use the sigmoid colon epiploica because the vaginal cuff was already very thin with a 2cm opening and there was a worry for a recurrent VVF if there was no intervening tissue.
This patient was a candidate for robotic-assisted laparoscopic repair of VVF because of her surgical history and risk for omental adhesions, medical history of endometriosis, and surgeon comfort and experience with robotic surgery. Further, because of robotic repair, the patient was able to be discharged home on the day of surgery with a follow-up one week post-operatively. Based on intraoperative findings, the location of the fistulas in the bladder dome would have been too high for vaginal repair. This case likely could have been completed with a laparoscopic approach. However, the patient’s history of endometriosis and two prior abdominal surgeries emphasized the need for enhanced visualization with the three-dimensional robotic console.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm.2019.12.01/coif). GK serves as an unpaid editorial board member of Gynecology and Pelvic Medicine from Nov 2019 to Oct 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bai SW, Huh EH, Jung DJ, et al. Urinary tract injuries during pelvic surgery: Incidence rates and predisposing factors. Int Urogynecol J Pelvic Floor Dysfunct 2006;17:360-4. [Crossref] [PubMed]
- Wong J, Bortoletto P, Tolentino J, et al. Urinary tract injury in gynecologic laparoscopy for benign indication: A systematic review. Obstet Gynecol 2018;131:100-8. [PubMed]
- Hillary C, Osman N, Hilton P, et al. The aetiology, treatment, and outcome of urogenital fistulae managed in well- and low-resourced countries: A systematic review. Eur Urol 2016;70:478-92. [Crossref] [PubMed]
- Agrawal V, Kucherov V, Bendana E, et al. Robotic-assisted laparoscopic repair of vesicovaginal fistula: A single center experience. Urology 2015;86:276-82. [Crossref] [PubMed]
- McKay E, Watts K, Abraham N. Abdominal approach to vesicovaginal fistula. Urol Clin North Am 2019;46:135-46. [Crossref] [PubMed]
- Ramphal S. Laparoscopic approach to vesicovaginal fistulae. Best Pract Res Clin Obstet Gynaecol 2019;54:49-60. [Crossref] [PubMed]
- Bora G, Singh S, Mavuduru R, et al. Robot-assisted vesicovaginal fistula repair: A safe and feasible technique. Int Urogynecol J 2017;28:957-62. [Crossref] [PubMed]
- Melomud O, Eichel B, Turbow B, et al. Laparoscopic vesicovaginal fistula repair with robotic reconstruction. Urology 2005;65:163-6. [Crossref] [PubMed]
- Gellhaus P, Bhandari A, Monn M, et al. Robotic management of genitourinary injuries from obstetric and gynaecological operations: A multi-institutional report of outcomes. BJU Int 2015;115:430-6. [Crossref] [PubMed]
- Sotelo R, Moros V, Clavijo R, et al. Surgery illustrated: Surgical atlas robotic repair of vesicovaginal fistula (VVF). BJU Int 2012;109:1416-34. [Crossref] [PubMed]
- Miklos J, Moore R, Chinthakanan O. Laparoscopic and robotic-assisted vesicovaginal fistula repair: A systematic review of the literature. J Minim Invasive Gynecol 2015;22:727-36. [Crossref] [PubMed]
Cite this article as: Counts RH, Lahham R, Kilic G, Lee TG. Robotic fistula repair of multiple vesicovaginal fistulas using sigmoid epiploica: case report. Gynecol Pelvic Med 2019;2:25.