
Role of vitamin D levels on the ovarian function and androgen profile in adolescents with polycystic ovarian syndrome
Introduction
Vitamin D is a hormone that acts via the vitamin D receptor (VDR) which belongs to the steroid/thyroid nuclear receptor superfamily (1). The presence of VDR has been found on the granulosa cells and the theca cells of the ovary (2). Animal studies have shown impaired folliculogenesis and lower estrogen levels in VDR null mice (3), indicating a role of vitamin D in reproductive functions. In humans, activated vitamin D or 1,25 di-hydroxyvitamin D has been shown to stimulate estrogen and progesterone production in the ovarian cells (3). Additionally, Vitamin D has been shown to stimulate aromatase activity in human skin fibroblasts, and it has been postulated that a similar mechanism may be responsible in the ovary as well (3,4).
A few clinical studies in adults have shown that Vitamin D supplementation can lower androgen levels, lower AMH levels, normalize the metabolic profile and regularize periods in women with polycystic ovarian syndrome (PCOS) (5,6) whereas certain studies have failed to show any effect on these parameters (7). There is a paucity of studies done in the adolescent age group.
Based on these findings, we hypothesized that vitamin D supplementation in patients with PCOS and vitamin D deficiency will regularize the periods and have a positive effect on their androgens. The objective of this study was to evaluate the effect of hypovitaminosis D on the ovarian function of adolescents with PCOS as assessed by clinical improvement (regularization of menstruation) and/or biochemical improvement (changes in serum LH/FSH levels and the serum androgen profile) by comparing the data before and after supplementation with vitamin D.
Methods
Study design
We performed a retrospective chart review of adolescent females 12–17 years of age who came to the Bellevue Pediatric endocrinology clinic from January 1, 2016 to August 20, 2018.
Females with vitamin D deficiency and irregular menstruation 2 years after menarche/or a diagnosis of PCOS in their problem list were included in the study as cases. And patients with vitamin D deficiency and obesity with regular menstruation were included as controls. The Rotterdam criteria was used to diagnose PCOS (any 2 of the following features: Irregular menstruation/chronic anovulation; biochemical or clinical evidence of hyperandrogenism and polycystic ovarian pathology). Vitamin D deficiency was defined as severe if the level was <10 ng/mL, mild-moderate for levels between 10–19 ng/mL and vitamin D insufficiency if the level was 20–29 ng/mL. A vitamin D level of 30 ng/mL or more was considered normal.
Exclusion criteria
Patients with irregular menstruation in the first 2 years after menarche, any patient already on vitamin D at the time of the initial evaluation, patients with any genetic syndrome, patients with any other chronic disease like hypothyroidism, hyperthyroidism, celiac disease, JIA, diabetes were excluded from the analysis.
All the patients were started on vitamin D supplementation after their first visit. They were started on 50,000 units weekly ×6–8 weeks followed by 2,000 units daily if the level was <20 ng/mL or 2,000 units daily if the level was >20 ng/mL. We relied on the medical records to assess compliance.
Data collection
Patient charts were reviewed to gather axiological data including BMI and ethnicity. The present and past medical history, menstrual history, medication history, birth history, family history was reviewed from the chart notes at each visit. Biochemical data including vitamin D (immunoassay), total and free testosterone (LC/MS), FSH and LH (immunoassays) was collected and when available, ultrasound pelvis was reviewed for the size of ovaries.
Follow up data at least 3 months after the initiation of vitamin D was recorded. When available, the 6-, 9-, 12-, 15-, 24- and 30-month follow up data with respect to menstrual history, vitamin D levels, androgen and gonadotropin profile was reviewed.
Data analysis
Descriptive analysis was made. For within the group, comparisons from pre–post vitamin D supplementation, and between group comparisons between cases and controls the paired t-test were used. P<0.05 are considered statistically significant.
Results
Twenty-four patients fulfilled the criteria to participate in the study. 17 patients qualified as cases and 7 patients were controls. The cases and controls were similar in regard to age, BMI, time since menarche, and family history of type 2 diabetes. Ethnic background of study patients included 64.7% Hispanic, 5.9% South Asian, 11.8% Caucasian and was unknown in 17.6%; controls included 71.4% Hispanic and 28.6% South Asian. All 24 patients were >2 years post-menarche (mean: 3.5±1.5 years for cases and 3.14±1.7 years for controls) (Figure 1).
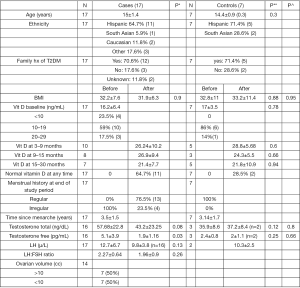
The clinical and biochemical characteristics of the cases and controls before and after vitamin D supplementation are shown in Figure 1. At baseline, all the cases had irregular menstruation and all the controls had regular periods. Thirteen/17 study patients had regular periods at the end of the study.
The mean (± SD) vitamin D level of the study patients at baseline was 16.2±6.4 ng/mL and that of controls was 17±3.5 ng/mL (P value: 0.78); 23.5% (4/17) of the cases presented with severe vitamin D deficiency, 59% (10/17) had mild-moderate vitamin D deficiency and 17.5% (3/17) had vitamin D insufficiency. None of the controls presented with vitamin D levels <10; 86% (6/7) had levels between 10–19 ng/mL and 14% (1/7) had levels between 20–29 ng/mL.
Out of the 17 study patients, 10 had vitamin D levels re-measured between 3–6 months, 8 patients had a repeat level done between 9–15 months and 7 between 15–30 months. Among the controls, 5 patients had a repeat Vitamin D level done between 3–6 months, 3 patients had a repeat level between 9–15 months and 5 patients had a repeat level between 15–30 months. All the patients had their vitamin D levels repeated at least once in the 30-month follow up period. There was no statistical difference between the vitamin D levels of the cases and controls at baseline, 3–9, 9–15 and 15–30 months after vitamin D supplementation. Sixty-five percent (11/17) cases and 28.5% of the controls (2/7) normalized their vitamin D levels at some point in the follow up period.
The mean initial total and free testosterone concentrations for the cases (n=16) were 57.68±22.8 ng/dL and 5.1±3.9 pg/mL respectively and that for the controls (n=3) was 35.9±8.6 ng/dL and 2.4±0.8 pg/mL respectively. There was no statistical difference between the initial total and free testosterone levels of the cases and controls (P value: 0.12 and 0.25 respectively). After vitamin D supplementation, the free testosterone concentration showed a significant decline from 5.1±3.9 to 1.9±1.16 pg/mL (P value: 0.03). The total testosterone level decreased to 43.2±23.25 ng/dL but failed to reach statistical significance (P value: 0.08). Since the controls had a normal Total and free testosterone concentration at baseline, repeat levels were not performed in a majority.
The mean initial LH level (n=17) of the study patients was 12.7±6.7 µ/L which decreased to 9.8±3.8 µ/L during the follow up period but was not statistically significant (P value: 0.13). The mean LH:FSH ratio also decreased from 2.27±0.64 to 1.96±0.9, but was also not statistically significant (P value: 0.26). Fourteen/17 study patients had a baseline pelvic ultrasound done. 50% of these patients had an ovarian volume >10 cc on either side.
Table 1 shows the menstrual pattern of the patients in the study group. 76.5% (13/17) of the patients regularized their periods during the 30-month follow up period whereas 4 patients continued to have irregular periods. All the patients were on vitamin D supplementation. Among the 13 patients with regular periods, 1 patient was also on metformin, 7 patients on oral contraceptive pills (OCPs), 4 on both metformin and OCPs and 1 required no additional intervention.
Table 1
| Variable | N | Before | N | After | |
|---|---|---|---|---|---|
| Menstrual history | 17 | 100% irregular | Regular | Irregular | |
| At the end of the study | 17 | 76.5% [13] | 23.5% [4] | ||
| Prior to/without OCPs | 17 | 11.8% [2] | 88.2% [15] | ||
| Patients with regular periods on vitamin D | 13 | ||||
| Metformin | 7.7% [1] | ||||
| Metformin + OCP | 30.8% [4] | ||||
| OCP | 53.8% [7] | ||||
| No intervention | 7.7% [1] | ||||
| Patients with irregular periods on vitamin D | 4 | ||||
| Metformin | 75% [3] | ||||
| OCP/OCP + metformin | 0 | ||||
| No intervention | 25% [1] | ||||
OCP, oral contraceptive pill.
Eleven of the thirteen patients (65%) with regular periods required OCPs. Of these eleven patients, 3 were started right at diagnosis, and the remaining 8 were started 6–27 months (mean: 12.75±8.2 months) after their initial visit. None of these 8 patients could get regular periods on vitamin D alone or on vitamin D + metformin.
Of the 11 study patients with a normal vitamin D level during the follow up period, 9 patients had regular periods. All 9 of these patients needed to be started on OCPs to regularize the periods.
There were only three patients who did not require metformin or OCPs. Only one patient’s periods regularized but the vitamin D level remained <20, in the other two patients the periods remained irregular. The vitamin D level increased to 34 ng/mL in one of them but remained in the mild-moderate deficiency range in the other.
Overall, 11/13 of the study patients with regular periods required OCPs ± metformin, 1 patient was on metformin and only one patient’s periods regularized with vitamin D supplementation alone but without normalization of their vitamin D.
Discussion
Numerous studies have tried to examine the vitamin D status of women with PCOS vs. obese controls. Some of these studies showed significantly lower baseline vitamin D levels in women with PCOS (8-12) whereas others have failed to show any such correlation (13-17). Wehr et al. demonstrated that adult women with PCOS have lower vitamin D levels compared to obese controls (9). Whereas, a study published by Kim et al. in 2014 failed to show any difference in the vitamin D levels of adult women with PCOS and obese controls (13). Two studies done in adolescents also failed to show a statistically significant difference between the vitamin D levels of patients with PCOS vs. controls (15,17). Like the latter three studies, our study failed to show any difference between the baseline vitamin D levels of adolescents with PCOS and Obese controls.
The impact of intervention with vitamin D supplementation in PCOS subjects has also been studied with mixed results. A randomized double-blind placebo controlled study done in India in 50 women with PCOS (18–45 years of age) in which they were given 12,000 units of vitamin D weekly for 12 weeks, showed that vitamin D supplementation alone was able to increase the percentage of patients with regular periods from 20% to 48% and was able to reduce the number of antral follicles by 40% (6). Similar findings were seen by Wahr et al. in 2011, where 23 out of 46 patients (18–46 years of age) with irregular menstruation were able to achieve regularization of the menses after vitamin D supplementation for 24 weeks (18). In our study, most of the patients were not on Vitamin D alone and required Metformin and/or OCPs to regularize menses. Only one patient on vitamin D alone managed to achieve regular periods. The vitamin D level in this patient increased from 6 to 16 ng/mL, but failed to normalize over a follow up period of 30 months. Another patient required Metformin to regularize the menses, however this patient’s vitamin D level remained <10 ng/mL, indicating that vitamin D did not have an impact on the regulation of menses in these two patients. In the other patients, since all of them were also on OCPs/Metformin, it is difficult to interpret the independent effect of vitamin D supplementation on menstruation.
Pal et al. published a before-after study in 12 obese women (19–39 years) with PCOS and found that vitamin D supplementation can lower the total testosterone and androstenedione levels (5). In our study, we saw a significant decrease in the free testosterone concentrations after vitamin D supplementation, however, since a lot of our patients were also on OCPs/metformin, it is hard to interpret the independent effect of vitamin D on the androgen profile; as OCPs are potent suppressors of ovarian androgen production. No change was noticed in the total testosterone, LH or the LH:FSH ratio after vitamin D supplementation. Our findings are similar to the findings in two meta-analyses published by Azadi-Yazdi et al. in 2017 and He et al. in 2015. Azadi-Yazdi reviewed the clinical trials looking at the effect of vitamin D supplementation on the androgenic profiles and found that the effect was not significant in randomized case-control studies (7). He et al. also failed to show an improvement in the hormonal profile of PCOS patients after supplementation with vitamin D (19).
A major limitation of our study is that it is a retrospective chart review, we had to rely on the medical records and were unable to gather all the data. Another limitation is that the sample size of our cohort is small which increases the risk of a type B error. Compliance with vitamin D is likely questionable. Many our patients were either on Metformin or OCPs or both, confounding our results. We were unable to analyze the androgen profiles prior to starting OCPs as they were not repeated in most of the patients. We did not have follow up pelvic ultrasounds to analyze a change in the size/distribution of the ovarian follicles after vitamin D supplementation. We did not have anti-Mullerian hormone (AMH) levels for our patients which strongly correlates with the number of ovarian follicles seen on ultrasound and can be used as a surrogate to evaluate for PCOS in adolescents (20,21). A decline in the AMH level with vitamin D may indicate an effect of vitamin D on ovarian function.
Conclusions
Thus, in conclusion, our study was unable to demonstrate an impact of vitamin D supplementation on regularizing menstruation or improving the androgen profile in adolescent girls with PCOS. A larger prospective study needs to be performed to evaluate the effect of vitamin D on the ovarian function.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm.2019.10.01/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional IRB of New York University School of Medicine (IRB number i18-01023). Since it is a retrospective chart review, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Drakopoulos P, van de Vijver A, Schutyser V, et al. The effect of serum vitamin D levels on ovarian reserve markers: a prospective cross-sectional study. Hum Reprod 2017;32:208-14. [PubMed]
- Kinuta K, Tanaka H, Moriwake T, et al. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology 2000;141:1317-24. [Crossref] [PubMed]
- Parikh G, Varadinova M, Suwandhi P, et al. Vitamin D regulates steroidogenesis and insulin-like growth factor binding protein-1 (IGFBP-1) production in human ovarian cells. Horm Metab Res 2010;42:754-7. [Crossref] [PubMed]
- Hodgins MB, Murad S. 1,25-Dihydroxycholecalciferol stimulates conversion of androstenedione into oestrone by human skin fibroblasts in culture. J Endocrinol 1986;110:R1-4. [Crossref] [PubMed]
- Pal L, Berry A, Coraluzzi L, et al. Therapeutic implications of vitamin D and calcium in overweight women with polycystic ovary syndrome. Gynecol Endocrinol 2012;28:965-8. [Crossref] [PubMed]
- Gupta T, Rawat M, Gupta N, et al. Study of Effect of Vitamin D Supplementation on the Clinical, Hormonal and Metabolic Profile of the PCOS Women. J Obstet Gynaecol India 2017;67:349-55. [Crossref] [PubMed]
- Azadi-Yazdi M, Nadjarzadeh A, Khosravi-Boroujeni H, et al. The Effect of Vitamin D Supplementation on the Androgenic Profile in Patients with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Clinical Trials. Horm Metab Res 2017;49:174-9. [Crossref] [PubMed]
- Joham AE, Teede HJ, Cassar S, et al. Vitamin D in polycystic ovary syndrome: Relationship to obesity and insulin resistance. Mol Nutr Food Res 2016;60:110-8. [Crossref] [PubMed]
- Wehr E, Pilz S, Schweighofer N, et al. Association of hypovitaminosis D with metabolic disturbances in polycystic ovary syndrome. Eur J Endocrinol 2009;161:575-82. [Crossref] [PubMed]
- Mahmoudi T, Gourabi H, Ashrafi M, et al. Calciotropic hormones, insulin resistance, and the polycystic ovary syndrome. Fertil Steril 2010;93:1208-14. [Crossref] [PubMed]
- Hahn S, Haselhorst U, Tan S, et al. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes 2006;114:577-83. [Crossref] [PubMed]
- Panidis D, Balaris C, Farmakiotis D, et al. Serum parathyroid hormone concentrations are increased in women with polycystic ovary syndrome. Clin Chem 2005;51:1691-7. [Crossref] [PubMed]
- Kim JJ, Choi YM, Chae SJ, et al. Vitamin D deficiency in women with polycystic ovary syndrome. Clin Exp Reprod Med 2014;41:80-5. [Crossref] [PubMed]
- Ganie MA, Marwaha RK, Nisar S, et al. Impact of hypovitaminosis D on clinical, hormonal and insulin sensitivity parameters in normal body mass index polycystic ovary syndrome women. J Obstet Gynaecol 2016;36:508-12. [Crossref] [PubMed]
- Bostanci EI, Ozler S, Yilmaz NK, et al. Serum 25-Hydroxy Vitamin D Levels in Turkish Adolescent Girls with Polycystic Ovary Syndrome and the Correlation with Clinical/Biochemical Parameters. J Pediatr Adolesc Gynecol 2018;31:270-3. [Crossref] [PubMed]
- Li HW, Brereton RE, Anderson RA, et al. Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metabolism 2011;60:1475-81. [Crossref] [PubMed]
- Sadhir M, Kansra AR, Menon S, Vitamin D. Deficiency among Adolescent Females with Polycystic Ovary Syndrome. J Pediatr Adolesc Gynecol 2015;28:378-81. [Crossref] [PubMed]
- Wehr E, Pieber TR, Obermayer-Pietsch B. Effect of vitamin D3 treatment on glucose metabolism and menstrual frequency in polycystic ovary syndrome women: a pilot study. J Endocrinol Invest 2011;34:757-63. [PubMed]
- He C, Lin Z, Robb SW, et al. Serum Vitamin D Levels and Polycystic Ovary syndrome: A Systematic Review and Meta-Analysis. Nutrients 2015;7:4555-77. [Crossref] [PubMed]
- Merhi Z, Doswell A, Krebs K, et al. Vitamin D alters genes involved in follicular development and steroidogenesis in human cumulus granulosa cells. J Clin Endocrinol Metab 2014;99:E1137-45. [Crossref] [PubMed]
- Baarends WM, Uilenbroek JT, Kramer P, et al. Anti-mullerian hormone and anti-mullerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology 1995;136:4951-62. [Crossref] [PubMed]
Cite this article as: Sodhi M, Shah BC. Role of vitamin D levels on the ovarian function and androgen profile in adolescents with polycystic ovarian syndrome. Gynecol Pelvic Med 2019;2:22.


