
Cancer of the uterine cervix: a narrative review
Introduction
Background
Uterine cervical cancer remains a significant global public health challenge. Its oncogenic mechanism is one of the most well-studied and is known to involve the human papillomavirus (HPV). The most common type of uterine cervical cancer is squamous cell carcinoma (SCC), which has been extensively researched and where prevention strategies have been devised. However, other, less common types of cancer require different treatment and prevention approaches (1).
Rationale and knowledge gap
Despite advancements in screening and vaccination, HPV continues to be a leading cause of cancer-related morbidity and mortality among women, particularly in low- and middle-income countries.
Objective
This review aims to provide a comprehensive overview of uterine cervical cancer, including its epidemiology, etiology, pathogenesis, clinical presentation, diagnostic approaches, treatment modalities, and preventive measures. We present this article following the Narrative Review reporting checklist (available at https://gpm.amegroups.com/article/view/10.21037/gpm-24-37/rc).
Methods
We searched MEDLINE, Google Scholar, and Scopus for original studies, narrative reviews, systematic reviews, and meta-analyses published in English. We also used references from the articles retrieved by our literature searches. The literature search was conducted without date limits and was focused on retrieving the most important and up-to-date knowledge. We used the search terms “cervical cancer” AND “human papillomavirus” AND “screening” OR “staging” OR “diagnosis” OR “microbiome” OR “treatment” OR “HPV DNA test” in our literature search (Table 1).
Table 1
| Item | Specification |
|---|---|
| Date of search | Search conducted between May 1 and July 31, 2024 |
| Databases and other sources searched | MEDLINE, Google Scholar, and Scopus |
| Search terms used | “cervical cancer” AND “human papillomavirus” AND “screening” OR “staging” OR “diagnosis” OR “microbiome” OR “treatment” OR “HPV DNA test” |
| Time frame | 1977–2024 |
| Inclusion criteria | Original studies, narrative reviews, systematic reviews, and meta-analyses published in English |
| Selection process | J.E. Jr and R.M.N.E. researched the articles and included those they considered the most representative for a narrative review |
Texts written in non-English languages, case reports, and opinions were excluded from our literature search.
Results and discussion
Histological classification
The World Health Organization (WHO) suggests that a histological classification of uterine cervical cancer should consider whether a tumor is associated with HPV (Table 2) (1).
Table 2
| Squamous epithelial tumors |
| Squamous cell carcinoma, HPV-associated |
| Squamous cell carcinoma, HPV-independent |
| Squamous cell carcinoma NOS |
| Glandular tumors |
| Adenocarcinoma NOS |
| Adenocarcinoma, HPV-associated |
| Adenocarcinoma, HPV-independent |
| Gastric type |
| Clear cell type |
| Mesonephric type |
| NOS |
| Endometrioid adenocarcinoma NOS |
| Carcinosarcoma NOS |
| Adenosquamous carcinoma |
| Mucoepidermoid carcinoma |
| Adenoid basal carcinoma |
| Carcinoma, undifferentiated, NOS |
| Mixed epithelial and mesenchymal tumors |
| Adenosarcoma |
| Germ cell tumors |
| Endodermal sinus tumor |
| Yolk sac tumor NOS |
| Choriocarcinoma NOS |
HPV, human papillomavirus; NOS, not otherwise specified.
Squamous cell carcinoma is the most common type of uterine cervical cancer (80%), followed by adenocarcinoma and adenosquamous carcinoma (approximately 20%). In high-income countries where effective cervical cancer prevention is available, the incidence of cervical adenocarcinoma has increased in the last three decades (2).
In rare cases, HPV-negative squamous cell carcinoma does not appear to have precancerous lesions. The morphology (of the biopsy sample) makes it impossible to differentiate between the two forms by immunohistochemistry, using p16ink4a as the surrogate marker of HPV. In contrast, adenocarcinoma has more frequent HPV-negative (denoted as p16ink4a negative) cases than squamous cell carcinoma. Indeed, these cases have a less favorable prognosis (1).
Epidemiology
Cervical cancer is the fourth most common cancer that affects women worldwide. There are an estimated 660,000 new cases and 350,000 deaths each year, at least 80% of which occur in third-world countries and are a significant cause of death in relatively young women (3,4).
Although there have been recent advances in surgery and chemotherapy for the treatment of this cancer, achieving cure rates greater than 80% in the early stages, regions with fewer resources are unable to prevent deaths. The highest incidence rates were observed in sub-Saharan Africa, Latin America, and Southeast Asia, reflecting disparities in access to preventive and healthcare services. In contrast, countries with well-established screening programs, such as the United States and Western Europe, have experienced a substantial decline in incidence and mortality rates over the past few decades (5).
We currently know that the most important factor in the development of cervical cancer is the persistence of high-risk HPV infections. In countries with a high incidence of cervical cancer, the frequency of persistent infection is approximately 20%, which is different from countries with a low incidence, where the persistence is approximately 5% (2).
Pathogenesis
HPV infection is the primary etiological factor in most cervical squamous cell carcinomas. The link between HPV and cervical cancer was demonstrated by zur Hausen approximately 50 years ago (6,7). HPV has been implicated in 99.7% of cervical squamous cell carcinoma cases worldwide (8).
HPV is a non-enveloped icosahedral virus (approximately 50–60 nm in diameter). Its genome consists of a single circular double-stranded DNA molecule with approximately 8,000 base pairs divided into open reading frames (ORF): an upstream regulatory region (URR), a late region (L1 and L2), and an early region (E1, E2, E4, E5, E6, and E7) (Figure 1). HPV types are defined by differences of 10% or more in L1. There are over 200 types (4,9-11).

The HPV viral types are classified as high or low risk. At least ten high-risk types are associated with cervical oncogenesis, namely, HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, and -59. On average, 70% of cervical cancers studied worldwide are associated with HPV-16 and -18 (12).
Cellular HPV infection occurs through contact. It can be skin-to-skin or skin-to-mucosa contact through fomites and the birth canal. Sexual transmission is by far the most common route of HPV infection (10).
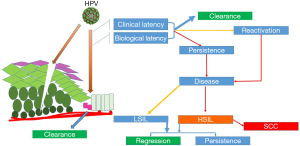
Viral infection can occur in immature cells observed at the squamous columnar junctions, such as junctional cells (13), reserve cells, or parabasal cells. Depending on the host’s immune response, the virus can be eliminated or may persist. The persistence of high-risk HPV is associated with an elevated risk of carcinoma (4). An HPV infection can cause an epithelial lesion (Figure 2) and may cause two pathological outcomes: a low-grade squamous intraepithelial lesion (LSIL) and a high-grade squamous intraepithelial lesion (HSIL). Only the latter can evolve into cervical cancer (in approximately 40% of HSIL cases) (14).
After accessing the cell, HPV, through the L1 protein, binds to heparan sulfate proteoglycans (HSPG). Thus, the L2 protein is exposed, and endocytosis and entry into the nucleus occur. Subsequently, gene replication and transcription occur. During this step, the viral genome can be episomalized or integrated into the genome (15). The function of each viral early ORF is shown in Table 3.
Table 3
| ORF | Function |
|---|---|
| E1 | Helps in the initiation and regulation of HPV replication |
| Acts as a helicase and recruits replication factors | |
| E2 | Aids in initiation and regulation of HPV replication |
| Enable the binding of E1 protein | |
| Disruption of E2 leads to cervical cancer progression | |
| Negative transcription regulator of E6 | |
| E4 | Changing of the cytokeratin network of the somatic cells |
| Involvement in arresting in the G2/M phase of the cell | |
| Amplification of viral replication | |
| Sustained activation of the mitogen-activated protein kinase (MAPK) signaling pathway | |
| Stabilization of the E2 protein | |
| E5 | E5 oncoprotein is associated with |
| • Cellular proliferation by activating the MAPK signaling pathway | |
| • Immune response evasion | |
| • Inhibition of apoptosis | |
| E6 | E6 oncoprotein promotes the ubiquitination and degradation of the tumor suppressor p53 via the proteasome and avoids cell death; promotes the expression of human telomerase reverse transcriptase (hTERT), which immortalizes the infected cell |
| E7 | E7 oncoprotein induces the degradation of the retinoblastoma protein (pRb) and deregulation of the E2F factor, cyclin A/CDK2, and cyclin E/Cdk2, inducing an uncontrolled cell proliferation |
ORF, open reading frame; HPV, human papillomavirus.
HPV integrates into the host genome, leading to the expression of viral oncoproteins E6 and E7 that inactivate the tumor suppressor proteins p53 and Rb, respectively. Disruption of cell cycle regulation results in uncontrolled cell proliferation and increases the potential for malignant transformation (11). Oncogenesis leads to the expression of marker proteins. The marker protein most frequently identified by immunohistochemistry is p16ink4a. This marker can differentiate between LSIL and HSIL in doubtful cases (14,16).
The p16ink4a locus encodes a tumor suppressor gene (TSG). The hypermethylation of TSG promoter regions results in impaired function, uncontrolled cell proliferation, and, eventually, cancer. DNA hypermethylation is the primary epigenetic event that can silence TSG transcription (17). Epigenetic mechanisms are essential for maintaining genetic expression patterns. Their disruption leads to altered gene function and malignant transformation, as in the hypermethylation explained above (18). In cells infected by high-risk HPV strains, the E7 protein inactivates the Rb protein with the release of E2F and activation of the cell cycle; this induces overexpression and accumulation of the p16ink4a protein in cells due to an inadequate feedback loop. It is important not to confuse the p16ink4a gene with the protein. The latter is where immunohistochemistry can assess its expression and has been used in practice to help diagnose HSIL. At the same time, it has been used along with Ki67 (p16/Ki67 double staining) in immunocytochemistry to triage cases that should be referred for colposcopy (19).
However, other associated factors are necessary for oncogenesis to occur. Cervical cancer is like chocolate cake. Chocolate is essential to making the cake, but we do not make a chocolate cake with chocolate alone. So, risk factors for cervical cancer include early onset of sexual activity, multiple sexual partners, immunosuppression (e.g., HIV infection), smoking, other sexually transmitted diseases, and long-term use of oral contraceptives. Socioeconomic factors also play a significant role, with lower socioeconomic status associated with higher risk due to limited access to screening and vaccination (20).
One essential co-factor in cervical cancer that has been studied recently is the vaginal microbiome. A classic study by Ravel et al. (21) classified the vaginal microbiome into five major groups of microbial communities. The first group is dominated by L. crispatus, the second by L. gasseri, the third by L. iners, the fourth by Lactobacillus, and the fifth by L. jensenii. Many studies have demonstrated the association of microbial community-3 and -4 with pre-invasive and invasive cervical lesions, respectively. Besides the vaginal microbiome, the metabolites present an influence on the HPV infection and progression of the lesion (22). An important finding is the relationship between isomer forms of lactic acid. The levogyre lactic acid seems to be associated with HSIL and invasive carcinoma (23).
Prevention
Considering that cervical cancer has a causal factor and a pre-invasive lesion, it is possible to have prevention strategies.
Prevention is divided into primary intervention, the greatest tool of which is the HPV vaccine, and secondary intervention, whose modalities include HPV DNA screening and the Papanicolaou (Pap) smear test (24).
HPV vaccine
Currently, recombinant protein vaccines against HPV have been used in many countries worldwide. They activate humoral immunity, leading to the production of neutralizing antibodies against HPV. The bond between antibodies and the virus prevents entry into the cell (25,26).
The method has shown high effectiveness and safety. Table 4 shows the characteristics of the vaccines and the vaccination schedule.
Table 4
| Components and vaccination schedule | Cervarix® | Gardasil® | Gardasil 9® |
|---|---|---|---|
| Virus-like particles | 16, 18 | 6, 11, 16, 18 | 6, 11, 16, 18, 31, 33, 45, 52, 58 |
| Adjuvant | ASO4 [0.5 mg aluminum hydroxide and 50 µg 3-O-desacyl-4'-monophosphoryl lipid A (MPL)] | 0.225 mg aluminum hydroxyphosphate sulfate | 0.5 mg aluminum hydroxyphosphate sulfate |
| Vaccination schedule | From 9 to 14 years old (including those who are 14 years old at the time of the first dose), Cervarix® can be administered with either a two- (0 and 6 months) or three-dose (0, 1 and 6 months) schedule | Individuals from 9 to 14 years can receive 2 doses of the vaccine (0 and 6 months) | Individuals from age 9 to 14 years can receive 2 doses of the vaccine (0 and 5 to 13 months) |
| In those over 14 years old up to 45 years, the vaccine must be given in 3 doses (0, 2 and 6 months) | In those over 14 to 45 years old, 3 doses are recommended (0, 2 and 6 months) | ||
| From 15 years of age onwards, only the three-dose schedule is recommended |
Cervarix® (GlaxoSmithKline Biologicals S.A., Wavre, Belgium); Gardasil® and Gardasil 9® (Merck Sharp & Dohme Corp, Rahway, NJ, USA). HPV, human papillomavirus.
A recent meta-analysis study observed that the HPV vaccine may have an adjuvant action in the surgical treatment of HSIL, reducing the recurrence rate of HPV-induced lesions (27). In addition, a recent multicenter study demonstrated the vaccine’s potential to protect hysterectomized women from developing lower genital tract lesions (28).
DNA-HPV test
The identification of HPV has been used as a screening strategy for precancerous lesions of the cervix. Recently, virus genotyping has improved this, allowing us to know the risk of infection-associated squamous lesions. Depending on the country, the test can be used alone or combined with cytological tests. The starting age for screening can be 25 or 30 years, and the screening ends around 64 years of age. Even with its high sensitivity, the DNA-HPV test is not effective if it does not have adequate coverage of the target population (29). Self-collection for DNA-HPV testing is suggested to achieve the much-desired screening coverage. A recent meta-analysis study involving 33 studies, with approximately 369,000 participants, demonstrated that self-collection for HPV testing in cervical cancer screening, whether at home or in a health service, increased the number of cases captured to an adequate level of treatment compared to standard screening (Pap smear) (30).
Papanicolaou test (Pap smear)
It is the most traditional screening test for cervical cancer prevention. The squamous-columnar junction is sampled, and the material can be spread on a glass slide (conventional cytology), or the brush of the collection device is detached and put in a vial with fixative liquid for subsequent processing (liquid-based cytology) (31). The cytological diagnosis is based on the Bethesda system for reporting cervical cytology (Table 5) (31-34). The procedure to be followed based on the cytological diagnosis is shown in Table 6.
Table 5
| Specimen type |
| Conventional smear (Pap smear)/liquid-based preparation/other |
| Specimen adequacy |
| Satisfactory for evaluation |
| Unsatisfactory for evaluation (specify reason) |
| General categorization (optional) |
| Negative for intraepithelial lesion or malignancy (NILM) |
| Epithelial cell abnormality |
| Refer to interpretation/result. Specify squamous/glandular as appropriate |
| Epithelial cell abnormalities |
| Squamous cell |
| ASC |
| ASC-US |
| Cannot rule out HSIL (ASC-H) |
| LSIL (encompassing HPV/mild dysplasia/CIN 1) |
| HSIL (encompassing moderate and severe dysplasia/CIS/CIN 2 and CIN 3) |
| With features suspicious for invasion (if suspected) |
| Squamous cell carcinoma |
| Glandular cell |
| Atypical |
| Endocervical cells (NOS or specify) |
| Endometrial cells (NOS or specify) |
| Glandular cells (NOS or specify) |
| Atypical |
| Endocervical cells (favor neoplastic) |
| Glandular cells (favor neoplastic) |
| Endocervical adenocarcinoma in situ |
| Adenocarcinoma |
| Endocervical |
| Endometrial |
| Extrauterine |
| NOS |
| Other malignant neoplasms (specify) |
ASC, atypical squamous cells; ASC-US, ASC of undetermined significance; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; HPV, human papillomavirus; CIN, cervical intraepithelial neoplasia; CIS, carcinoma in situ; NOS, not otherwise specified.
Table 6
| Diagnosis | Approach |
|---|---|
| ASC-US (atypical squamous cells of undetermined significance) | DNA-HPV test, follow-up (6 months and 1 year), or colposcopy, depending on the country and availability of tools |
| ASC-H (atypical squamous cells, cannot rule out HSIL) | Colposcopy |
| LSIL (low-grade squamous intraepithelial lesion) | Colposcopy (follow-up may be suggested in some places) |
| HSIL (high-grade squamous intraepithelial lesion) | Colposcopy |
| Squamous cell carcinoma | Colposcopy |
| Atypical glandular cell (AGC) | Colposcopy |
| Adenocarcinoma in situ | Colposcopy |
| Adenocarcinoma | Colposcopy |
HPV, human papillomavirus.
Diagnosis
Cervical cancer, similar to precancerous lesions, is asymptomatic in most cases. In more advanced cases, bloody and foul-smelling discharge may occur. Usually, the diagnosis is made during a routine gynecological examination, where the results of an oncotic cytology and/or an HPV DNA test are positive, and the woman is referred for colposcopy. Colposcopy is an excellent method for guiding the correct sampling location for a correct biopsy diagnosis. During colposcopy, some findings may suggest an invasive squamous lesion, such as thick acetowhite epithelium, ulcerative lesions, exophytic tumors, friable lesions, easy bleeding, and coarse atypical vessels (35).
The most crucial function of colposcopy is to identify and biopsy pre-invasive lesions. In cases where HSIL must be differentiated from a mimicking lesion, such as immature squamous metaplasia (Figure 3) and atrophy, and in cases of a former intraepithelial neoplasia grade 2 (CIN2), immunohistochemistry for detecting p16ink4a is suggested. If the results are positive, the final diagnosis is HSIL (Table 7, Figure 3) (14).
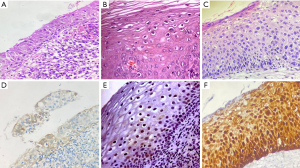
Table 7
| Lesion | Criteria |
|---|---|
| LSIL | Squamous or metaplastic cells with abnormal nuclear features (increased nuclear size, irregular nuclear membranes, and increased nuclear-to-cytoplasmic ratios) |
| There is little cytoplasmic maturation in the lower third of the epithelium, but maturation begins in the middle third and is relatively normal in the upper third | |
| Mitotic figures are limited to the lower one-third of the epithelium | |
| The presence of the diagnostic cytopathic effect of HPV (koilocytosis) | |
| HSIL | Squamous or metaplastic squamous cells with abnormal nuclear features (increased nuclear size, irregular nuclear membranes, and increased nuclear-to-cytoplasmic ratios accompanied by mitotic figures) |
| Little or no cytoplasmic differentiation in the middle third and superficial thirds of the epithelium | |
| Mitotic figures may be found in the middle and/or superficial thirds of the epithelium |
LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; HPV, human papillomavirus.
Staging of cervical cancer
Cervical cancer is staged to define its treatment and prognosis. Management strategies are suggested depending on the staging. The International Federation of Gynecology and Obstetrics (FIGO) suggested staging in 2018, and this was later updated in 2021 to include clinical, radiological, and pathological aspects, with the critical observation that imaging and pathology can be used to supplement clinical findings concerning tumor size and extent in all stages (Table 8) (36).
Table 8
| Stage | Description |
|---|---|
| I | The carcinoma is strictly confined to the cervix (extension to the uterine corpus should be disregarded) |
| IA | Invasive carcinoma that can be diagnosed only by microscopy, with maximum depth of invasion ≤5 mm |
| IA1 | Measured stromal invasion ≤3 mm in depth |
| IA2 | Measured stromal invasion >3 and ≤5 mm in depth |
| IB | Invasive carcinoma with measured deepest invasion >5 mm (greater than stage IA); lesion limited to the cervix uteri with size measured by maximum tumor diameter |
| IB1 | Invasive carcinoma >5 mm depth of stromal invasion and ≤2 cm in greatest dimension |
| IB2 | Invasive carcinoma >2 and ≤4 cm in greatest dimension |
| IB3 | Invasive carcinoma >4 cm in greatest dimension |
| II | The carcinoma invades beyond the uterus, but has not extended onto the lower third of the vagina or to the pelvic wall |
| IIA | Involvement limited to the upper two-thirds of the vagina without parametrial involvement |
| IIA1 | Invasive carcinoma ≤4 cm in greatest dimension |
| IIA2 | Invasive carcinoma >4 cm in greatest dimension |
| IIB | With parametrial involvement but not up to the pelvic wall |
| III | The carcinoma involves the lower third of the vagina and/or extends to the pelvic wall and/or causes hydronephrosis or nonfunctioning kidney and/or involves pelvic and/or para-aortic lymph nodes |
| IIIA | The carcinoma involves the lower third of the vagina, with no extension to the pelvic wall |
| IIIB | Extension to the pelvic wall and/or hydronephrosis or nonfunctioning kidney (unless known to be due to another cause) |
| IIIC | Involvement of pelvic and/or para-aortic lymph nodes (including micrometastases), irrespective of tumor size and extent (with r and p notations) |
| IIIC1 | Pelvic lymph node metastasis only |
| IIIC2 | Para-aortic lymph node metastasis |
| IV | The carcinoma has extended beyond the true pelvis or has involved (biopsy proven) the mucosa of the bladder or rectum. A bullous edema, as such, does not permit a case to be allotted to stage IV |
| IVA | Spread of the growth to adjacent pelvic organs |
| IVB | Spread to distant organs |
FIGO, International Federation of Gynecology and Obstetrics.
Treatment
Pre-invasive lesions
Excision and histopathological studies are recommended for a pre-invasive lesion. Depending on the characteristics of the lesion observed via colposcopy, excision can be achieved through high-frequency surgery or classic conization (14).
Staging conditions for conduction
Table 9 demonstrates this behavior in general terms. We suggest consulting Bhatla et al. [2021] (36) for more details.
Table 9
| Stage | Treatment |
|---|---|
| Stage IA1 | Conization or hysterectomy if the woman has completed childbearing |
| Stage IA2 | Type 2 radical hysterectomy (ligation of the uterine artery where it crosses the ureter, although a vaginal cuff is not necessary) with pelvic lymphadenectomy |
| Stage IB–IVA | Chemoradiotherapy |
| Radical hysterectomy with pelvic lymphadenectomy may be indicated in Stage IB1–IIA1 | |
| Primary pelvic exenteration may be considered for Stage IVA |
Stage IVB/distant metastases
Chemotherapy
Strengths and limitations
Narrative reviews have limitations due to their subjectivity in the search for articles. However, their strength lies in using important articles and updated guidelines.
Conclusions
Cervical cancer is perhaps the most studied neoplasm. Its oncogenesis mechanisms, as well as prevention strategies, are well known. However, it is still a cancer that kills many women around the world. Our current review provides up-to-date knowledge of the etiology, prevention, diagnosis, and treatment of uterine cervical cancer, which will be vitally important for healthcare professionals in this field.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://gpm.amegroups.com/article/view/10.21037/gpm-24-37/rc
Peer Review File: Available at https://gpm.amegroups.com/article/view/10.21037/gpm-24-37/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.com/article/view/10.21037/gpm-24-37/coif). J.E. Jr is currently a speaker for MSD’s 9-valent HPV vaccine for Brazil. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO Classification of Tumours Editorial Board. WHO Classification of Female Genital Tumours. 5th ed. International Agency for Research on Cancer (IARC); 2020.
- Abu-Rustum NR, Yashar CM, Arend R, et al. NCCN Guidelines® Insights: Cervical Cancer, Version 1.2024. J Natl Compr Canc Netw 2023;21:1224-33. [Crossref] [PubMed]
- WHO. Cervical cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer. Accessed on 06/19/2024.
- Golia D’Augè T, Cuccu I, Etrusco A, et al. State of the art on HPV-related cervical lesions Ital J Gynaecol Obstet 2024;36:135-7. (letter). [Crossref]
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- zur Hausen H. Human papillomaviruses and their possible role in squamous cell carcinomas. Curr Top Microbiol Immunol 1977;78:1-30. [Crossref] [PubMed]
- zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology 2009;384:260-5. [Crossref] [PubMed]
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12-9. [Crossref] [PubMed]
- Bravo IG, Félez-Sánchez M. Papillomaviruses: Viral evolution, cancer and evolutionary medicine. Evol Med Public Health 2015;2015:32-51. [Crossref] [PubMed]
- Hernández-Silva CD, Ramírez de Arellano A, Pereira-Suárez AL, et al. HPV and Cervical Cancer: Molecular and Immunological Aspects, Epidemiology and Effect of Vaccination in Latin American Women. Viruses 2024;16:327. [Crossref] [PubMed]
- Bhattacharjee R, Das SS, Biswal SS, et al. Mechanistic role of HPV-associated early proteins in cervical cancer: Molecular pathways and targeted therapeutic strategies. Crit Rev Oncol Hematol 2022;174:103675. [Crossref] [PubMed]
- Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol 2009;10:321-2. [Crossref] [PubMed]
- Herfs M, Yamamoto Y, Laury A, et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci U S A 2012;109:10516-21. [Crossref] [PubMed]
- Darragh TM, Colgan TJ, Cox JT, et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med 2012;136:1266-97. [Crossref] [PubMed]
- Graham SV. Keratinocyte Differentiation-Dependent Human Papillomavirus Gene Regulation. Viruses 2017;9:245. [Crossref] [PubMed]
- Eleutério J Jr, Giraldo PC, Gonçalves AK, et al. Prognostic markers of high-grade squamous intraepithelial lesions: the role of p16INK4a and high-risk human papillomavirus. Acta Obstet Gynecol Scand 2007;86:94-8. [Crossref] [PubMed]
- Skvortsova K, Stirzaker C, Taberlay P. The DNA methylation landscape in cancer. Essays Biochem 2019;63:797-811. [Crossref] [PubMed]
- Han YD, Wang XB, Cui NH, et al. Associations of P16INK4a promoter hypermethylation with squamous intra-epithelial lesion, cervical cancer and their clinicopathological features: a meta-analysis. Oncotarget 2017;8:1871-83. [Crossref] [PubMed]
- Yu L, Fei L, Liu X, et al. Application of p16/Ki-67 dual-staining cytology in cervical cancers. J Cancer 2019;10:2654-60. [Crossref] [PubMed]
- Bosch FX, Lorincz A, Muñoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002;55:244-65. [Crossref] [PubMed]
- Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 2011;108:4680-7. [Crossref] [PubMed]
- Łaniewski P, Ilhan ZE, Herbst-Kralovetz MM. The microbiome and gynaecological cancer development, prevention and therapy. Nat Rev Urol 2020;17:232-50. [Crossref] [PubMed]
- de Magalhães CCB, Linhares IM, Masullo LF, et al. Comparative measurement of D- and L-lactic acid isomers in vaginal secretions: association with high-grade cervical squamous intraepithelial lesions. Arch Gynecol Obstet 2022;305:373-7. [Crossref] [PubMed]
- World Health Organization. WHO guidance note: comprehensive cervical cancer prevention and control: a healthier future for girls and women. 2013. Available online: https://www.who.int/publications/i/item/9789241505147. Accessed on 06/19/2024.
- Yousefi Z, Aria H, Ghaedrahmati F, et al. An Update on Human Papilloma Virus Vaccines: History, Types, Protection, and Efficacy. Front Immunol 2021;12:805695. [Crossref] [PubMed]
- Wang R, Pan W, Jin L, et al. Human papillomavirus vaccine against cervical cancer: Opportunity and challenge. Cancer Lett 2020;471:88-102. [Crossref] [PubMed]
- Di Donato V, Caruso G, Petrillo M, et al. Adjuvant HPV Vaccination to Prevent Recurrent Cervical Dysplasia after Surgical Treatment: A Meta-Analysis. Vaccines (Basel) 2021;9:410. [Crossref] [PubMed]
- Bogani G, Sopracordevole F, Ciavattini A, et al. HPV-related lesions after hysterectomy for high-grade cervical intraepithelial neoplasia and early-stage cervical cancer: A focus on the potential role of vaccination. Tumori 2024;110:139-45. [Crossref] [PubMed]
- Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 2013;17:S1-S27. [Crossref] [PubMed]
- Yeh PT, Kennedy CE, de Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Glob Health 2019;4:e001351. [Crossref] [PubMed]
- Nayar R, Wilbur DC. The Bethesda System for Reporting Cervical Cytology: Definitions, Criteria, and Explanatory Notes. 3rd ed. Switzerland: Springer International Publishing; 2015.
- Perkins RB, Guido RS, Castle PE, et al. ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 2020;24:102-31. Erratum in: J Low Genit Tract Dis 2020;24:427. [Crossref] [PubMed]
- Sundström K, Lu D, Elfström KM, et al. Follow-up of women with cervical cytological abnormalities showing atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion: a nationwide cohort study. Am J Obstet Gynecol 2017;216:48.e1-48.e15. [Crossref] [PubMed]
- Ministry of Health of Brazil. José Alencar Gomes da Silva National Cancer Institute (INCA). Diretrizes Brasileiras para o Rastreamento do Câncer do Colo do Útero. Coordenação de Prevenção e Vigilância. 2nd ed. Rio de Janeiro: INCA; 2016.
- Burness JV, Schroeder JM, Warren JB. Cervical Colposcopy: Indications and Risk Assessment. Am Fam Physician 2020;102:39-48. [PubMed]
- Bhatla N, Aoki D, Sharma DN, et al. Cancer of the cervix uteri: 2021 update. Int J Gynaecol Obstet 2021;155:28-44. [Crossref] [PubMed]
Cite this article as: Eleutério J Jr, Cavalcante DIM, Maia GH, Eleutério RMN. Cancer of the uterine cervix: a narrative review. Gynecol Pelvic Med 2024;7:33.


