
Minimally invasive cytoreduction for advanced-stage ovarian cancer—a narrative review
Introduction
High grade ovarian cancer is the most lethal cancer affecting the female reproductive tract, and contributes to approximately 4.5% of all cancer-related deaths in the United States in 2024 (1). Mostly asymptomatic in its early stages and lacking efficient screening modalities, a substantial proportion (80–85%) of ovarian cancer cases are diagnosed at an advanced stage (2). Conventional surgical management of ovarian cancer typically involves a comprehensive debulking or cytoreduction that comprises total hysterectomy, bilateral salpingo-oophorectomy, peritoneal washings, biopsies or removal of peritoneal and diaphragmatic surfaces, omentectomy, and frequently bowel resection and retroperitoneal lymph node sampling in the pelvic and/or para-aortic regions through an extensive vertical midline laparotomy incision. The primary objectives of this surgical intervention are accurate disease diagnosis and staging, along with achieving complete cytoreduction to eliminate all visible disease (2). While traditionally the cytoreductive surgery was through abdominal midline incision, minimally invasive (MIS) cytoreductive surgery is gaining popularity and needs to be validated with outcome data. We aim to review the main evidence pertaining to minimally invasive cytoreductive surgery for ovarian cancer. We present this article in accordance with the Narrative Review reporting checklist (available at https://gpm.amegroups.com/article/view/10.21037/gpm-24-25/rc).
Methods
This narrative review did not follow a systematic review approach. We reviewed the pertinent studies regarding MIS for advanced ovarian cancer and included all study designs. We have searched the PubMed database for all original studies published with the MeSH terms “minimally invasive surgical procedures” and “ovary neoplasm”. We limited our search to original articles in English language. We excluded reviews, case series and other publications which are non-original articles (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 21 April, 2024 |
| Databases searched | PubMed |
| Search terms used | Minimally invasive surgical procedures, ovary neoplasm |
| Timeframe | Up to April 2024 |
| Inclusion criteria | Original articles in English language |
| Selection process | Both authors (G.L., W.G.) selected the studies for the narrative review |
Literature review
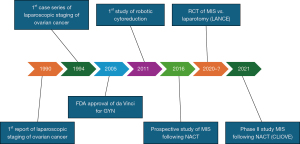
The first report of laparoscopic staging of ovarian cancer was published in 1990 (3) (Figure 1). Progressively, laparoscopy emerged as a viable approach for staging early ovarian cancer, with the first series published in 1994 comprising nine patients who underwent lymphadenectomies up to the level of the renal veins and who were discharged an average of 2.8 days after surgery (4). Subsequently, the advantages of MIS over laparotomy have been proposed, including superior intraoperative visualization and smaller incisions, reduced blood loss, decreased postoperative complications such as wound infections and ileus, shorter hospitalization time, and faster recovery (5-8). However, laparoscopy also presents its own limitations, often including lack of depth perception, camera instability, dependence on skilled surgical assistance, counter-intuitive hand motion, limited range of motion, non-wristed instrumentation, resistance at the anterior abdominal wall, and prolonged learning curves (9). The introduction of the da Vinci computer-assisted surgical system (Intuitive Surgical, Sunnyvale, CA, USA), approved by the Food and Drug Administration (FDA) in 2005 for gynaecological procedures, marked a significant advancement in surgical technology (10). The integration of robotic technology into gynaecological oncological surgery has transformed surgical practices and approaches to managing many gynaecological malignancies (11).
Robot-assisted surgery offers the advantages of standard laparoscopy while addressing many of its limitations. It provides three-dimensional (3D) immersion vision, ergonomic and intuitive controls, improved dexterity, tremor-cancelling software for enhanced precision, and wristed instruments that mimic human hand movements (12). The smaller instruments and magnified operative field enable greater accuracy, particularly beneficial for precise dissection and complex surgical procedures (13).
The first published study of robotic-assisted cytoreduction for ovarian cancer compared 25 patients operated via robotic approach in the period 2005–2008, to patients treated by laparoscopy and laparotomy, matched by body mass index (BMI), type and number of surgical procedures (14). In this study, Magrina and his colleagues showed that robotic surgery for advanced ovarian cancer was associated with longer operative time—mean operative time of 314.8 vs. 253.8 minutes for laparoscopy and 260.7 minutes for laparotomy. Blood loss was less via the robotic approach 164.0 vs. 266.7 mL in laparoscopy and 1,307.0 mL in laparotomy, while 3-year survival was similar in all groups 67.1% for robotics, 75.6% for laparoscopy and 66.0% for laparotomy (P=0.08). Length of stay was significantly shorter in robotics (4.2 days) when compared to laparotomy (9.4 days). Although selection bias was a significant concern, the study was reassuring and the conclusions of the authors was that robotics and laparoscopy are preferable for highly selected patients, with laparotomy being preferable when two or more major procedures are necessary (in addition to conventional cytoreduction). Most importantly, survival was not statistically affected by the type of surgical approach, although selection bias remains the main limitation of this study.
A following study by Feuer et al. compared robotic surgery to conventional laparotomy for advanced ovarian cancer during the period 2008–2012 (15). In this retrospective study, the robotic cohort contained 63 patients and showed similar findings to the aforementioned study regarding operative time, blood loss and postoperative stay, underscoring the benefits of robotic surgery in these domains. Importantly, residual disease rates were similar between robotic route and laparotomy and survival rates at one year, although short, were equivalent for all cases. The study concluded that the robotic approach for ovarian cancer, including patients treated with neoadjuvant chemotherapy (NACT), is feasible and effective.
Another study evaluated 47 cytoreductive surgeries in patients with advanced ovarian cancer; among those, ten robotic procedures (5). Their rate of intraoperative transfusion was 63% for laparotomy and none for robotic cytoreduction. The blood loss was lower, and the hospital stay was shorter for robotic surgery compared to laparotomy. Degree of cytoreduction, complication rates, and surgical times were similar for robotic surgery and laparotomy.
Overall, these pioneering studies suggested that the utilization of robotic surgery seemed in selected cases to allow sufficient debulking and appropriate exploration of both the abdomen and pelvis, and resulted in lymph node counts and omentectomy yields comparable to those achieved through traditional laparotomy. Patients undergoing robotic surgery exhibited swifter postoperative recovery without compromising survival rates in the selected patients.
Primary cytoreduction before initiation of chemotherapy has been the standard of care for patients with advanced ovarian cancer. The EORTC-GCG/NCIC-CTG randomized trial published in the New England Journal of Medicine in 2010 (16), demonstrated that NACT followed by interval cytoreduction surgery was not inferior to primary cytoreductive surgery followed by chemotherapy in patients with stage IIIC or IV ovarian carcinoma. This was further consolidated in other studies (17-20) and in a meta-analysis of randomised controlled trials. Following those trials and the safety data provided, the rate of utilization of NACT increased significantly, coupled with the increasing use of laparoscopy and robotic surgery (21). In a prospective multi-center Italian study, the “MISSION” trial (22), 30 patients were included who underwent NACT and MIS interval cytoreduction. Only four of these MIS were performed using the robotic platform. This feasibility study concluded that in patients with clinically complete response to NACT, it is feasible and safe to perform laparoscopy and robotic cytoreduction in terms of perioperative outcomes, psycho-oncological impact, and survival data. Importantly, a phase II prospective French multicenter non-randomized trial [the CILOVE study (23)] studied the feasibility and safety of laparoscopic approach after NACT among selected chemosensitive patients with advanced ovarian cancer. Thirty-two patients underwent laparoscopic cytoreduction with a 9.4% (3/32) conversion rate. All except one patient had optimal cytoreduction. This prospective study concluded that interval ovarian cytoreduction by a laparoscopic approach is safe and feasible for patients with a favorable response to chemotherapy.
The ongoing LANCE trial will be the first to provide level I evidence on oncologic safety of MIS after NACT (24). This international, prospective, randomized, multicenter, non-inferiority phase III trial compares MIS versus laparotomy for advanced-stage high-grade epithelial ovarian cancer following NACT and normalization of CA-125, with disease free survival as primary endpoint. The results of this trial may be practice-changing if non-inferiority of MIS cytoreduction will be demonstrated.
Awaiting Level I evidence, retrospective studies and meta-analyses may provide valuable evidence. A retrospective audit of cases operated during 2011–2016 included 29 patients (25) undergoing MIS, and only one case (3.4%) was converted to laparotomy due to disease burden not amenable to robotic cytoreduction. Overall, 66% patients underwent a R0 cytoreduction, and 28% an optimal (<1 cm) cytoreduction. Of note, only 7% underwent a suboptimal cytoreduction. The median progression-free survival was 21.2 months, and the median overall survival was 39.7 months. A study reporting 12 patients undergoing robotic-assisted interval cytoreductive surgery published in 2020 (26) showed that optimal cytoreduction could be achieved in all 12 patients, and complete cytoreduction surgery was achieved in 75% of patients. The estimated mean blood loss was 100 mL, and the median hospital stay was 2 days. Our group compared 57 patients who underwent robotic interval cytoreduction to 34 patients who had interval cytoreduction by laparotomy (27). All patients selected for interval robotic cytoreduction following NACT had resolution of ascites, a reduction of CA-125 by at least 80%, and imaging suggesting the potential to achieve complete interval cytoreduction. No residual disease (R0) was achieved in 82% of cases and the remaining 18% achieved optimal cytoreduction with <1 cm residual disease. A meta-analysis of the retrospective data and the MISSION study was published in 2021, including a total of 102 patients (28). Conversion rate to laparotomy was 9.2%. Complete cytoreduction rate (R0) was achieved in 76.5%, and another 21.5% had residual disease ≤1 cm. The progression-free survival ranged from 20.6 to 21.2 months and the median overall survival varied from 39.7 to 47.2 months.
Another retrospective study comparing 43 robotic interval cytoreductions to 50 laparotomy interval cytoreductions was published in 2021 (29). Complete cytoreduction rate was not influenced by surgical modality (52% in laparotomy vs. 63% in robotic surgery). Progression-free survival and overall survival did not differ between study groups, with progression-free survival of 15.4 vs. 16.7 months, and overall survival of 38.2 vs. 35.6 months.
The most recent study published in 2022 (30) used data from a single surgeon in a single center with strict selection criteria. With a median follow-up time of 52 months in the robotic interval cytoreduction group, 42.9% of patients experienced a recurrence, which was a similar rate to the recurrence for laparotomy interval cytoreduction (45%). A summary of the studies is presented in Table 2.
Table 2
| Study | Duration | Number of surgeries/studies | Methodology | Procedure | Outcomes | Results |
|---|---|---|---|---|---|---|
| Querleu et al. (4) | 1991–1993 | 9 | Descriptive | LSC | Infra-renal LND | Successful |
| Postoperative stay | 2.8 days | |||||
| Nezhat et al. (5) | 2008–2012 | 83 | Comparative | LSC vs. RAS vs. laparotomy | Blood loss, length of stay, surgery length, postoperative complications | LSC is comparable to RAS and not inferior to laparotomy |
| Ghezzi et al. (6) | 2003–2007 | 34 | Comparative | LSC vs. laparotomy | Blood loss, operative time, hospital stay, complications | LSC has longer surgical time and shorter hospital stay |
| Park et al. (7) | 2001–2006 | 36 | Comparative | LSC vs. laparotomy | Blood loss, operative time, time to chemotherapy, postoperative stay | LSC has less blood loss and shorter hospital stay |
| Falcetta et al. (8) | 1990–2016 | 7 case-control, 8 cohort | Cochrane Review | LSC vs. laparotomy | – | No high-level evidence of risk and benefits of LSC over laparotomy |
| Magrina et al. (14) | 2005–2008 | 25 | Comparative | LSC vs. RAS vs. laparotomy | Blood loss, operative time, hospital stay, complications, overall survival | Survival is not affected by type of surgical approach |
| Feuer et al. (15) | 2008–2012 | 89 | Comparative | RAS vs. laparotomy | Blood loss, hospital stay, complications, residual disease, disease-free survival, overall survival | Similar survival and recurrence rates at 1-year |
| Gueli Alletti et al. (22) | 2013–2015 | 30 | Phase II | LSC & RAS | Interval cytoreductive surgery | MIS is feasible and safe in terms of perioperative outcomes, psycho-oncological impact, and survival rate |
| Ackroyd et al. (25) | 2011–2016 | 29 | Descriptive | RAS | Interval cytoreductive surgery | 66% R0 |
| Carbajal-Mamani et al. (26) | 2017–2018 | 23 | Descriptive | RAS | Interval cytoreductive surgery | 75% R0 |
| Abitbol et al. (27) | 2008–2014 | 91 | Comparative | RAS vs. laparotomy | Interval cytoreductive surgery | 82% R0 in RAS. Survival is not affected by route of surgery |
| Zhang et al. (29) | 2011–2018 | 93 | Comparative | RAS vs. laparotomy | Interval cytoreductive surgery | Disease-free survival and overall survival are comparable. R0 rate is similar in both groups |
| Van Trappen et al. (30) | 2015–2021 | 96 | Comparative | RAS vs. laparotomy | Staging surgery | Similar oncological outcomes. Shorter hospital stay in RAS |
| Interval cytoreductive surgery |
LND, lymph node dissection; LSC, laparoscopy; MIS, minimally invasive surgery; RAS, robotic-assisted surgery.
Bibliometric studies are the quantitative analysis of publications, citations, and other bibliographic data to understand patterns of academic activity within a specific field or across disciplines. These studies may provide insights into trends and impact of studies. Previous studies have underlined the importance of research patterns in MIS in gynecology (10). The temporal trend of number of publications pertaining to MIS in ovarian cancer is presented in Figure 2. Examining the publications regarding MIS in ovarian cancer may signal the rising impact of MIS in advanced ovarian cancer. The number of patients reported to undergo MIS for ovarian cancer is presented in Figure 3. There is a constant, yet slow increase in the number of patients reported (R2=0.3352).
Our review has some limitations. Of note, we cannot account for all technical details in the studies discussed pertaining to robotic-assisted surgery (e.g., types of platforms use, ports position and docking methods for the cytoreduction surgeries). This information is of importance to surgeons, and furthermore, this may change over time and on case-to-case basis. It is possible that some surgeons will state that more data is needed to recommend robotic surgery in advanced ovarian cancer therapy.
The primary critic concerning robotic surgery in advanced ovarian cancer revolves around the difficulty in thoroughly exploring and palpating all four quadrants of the abdomen, particularly the upper abdomen. This challenge may possibly result in inaccuracies in assessing the tumor burden and residual disease. On the other hand, the lower morbidity and earlier resumption of systemic treatments might balance the lack of complete visualization and palpation. How this less traumatic surgical experience will affect patient’s health span and possibly lifespan in the era of personalized medicine and innovative systemic treatments remains to be determined.
Most data presented in this review are retrospective and the ongoing LANCE trial results should shed more light on the oncological safety of MIS cytoreduction. Until level 1 evidence will be presented, further prospective trials and retrospective audits of large cohorts will provide more data to evaluate the role of the MIS approach.
Conclusions
There is value to engage in open discussions with the patient to determine the most suitable therapeutic approach for each unique clinical scenario that will provide the best outcome for the individual patient and determine the goal of care with the patient.
Acknowledgments
Funding: This research was made possible by
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://gpm.amegroups.com/article/view/10.21037/gpm-24-25/rc
Peer Review File: Available at https://gpm.amegroups.com/article/view/10.21037/gpm-24-25/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.com/article/view/10.21037/gpm-24-25/coif). W.H.G. serves as an unpaid editorial board member of Gynecology and Pelvic Medicine from June 2024 to December 2025. W.H.G. also reports payment from Intuitive Surgical, Merck and GSK. G.L. is supported by a grant of the Israel Cancer Research Fund. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12-49. [Crossref] [PubMed]
- Lheureux S, Gourley C, Vergote I, et al. Epithelial ovarian cancer. Lancet 2019;393:1240-53. [Crossref] [PubMed]
- Reich H, McGlynn F, Wilkie W. Laparoscopic management of stage I ovarian cancer. A case report. J Reprod Med 1990;35:601-4; discussion 604-5. [Crossref] [PubMed]
- Querleu D, LeBlanc E. Laparoscopic infrarenal paraaortic lymph node dissection for restaging of carcinoma of the ovary or fallopian tube. Cancer 1994;73:1467-71. [Crossref] [PubMed]
- Nezhat FR, Finger TN, Vetere P, et al. Comparison of perioperative outcomes and complication rates between conventional versus robotic-assisted laparoscopy in the evaluation and management of early, advanced, and recurrent stage ovarian, fallopian tube, and primary peritoneal cancer. Int J Gynecol Cancer 2014;24:600-7. [Crossref] [PubMed]
- Ghezzi F, Cromi A, Uccella S, et al. Laparoscopy versus laparotomy for the surgical management of apparent early stage ovarian cancer. Gynecol Oncol 2007;105:409-13. [Crossref] [PubMed]
- Park JY, Bae J, Lim MC, et al. Laparoscopic and laparotomic staging in stage I epithelial ovarian cancer: a comparison of feasibility and safety. Int J Gynecol Cancer 2008;18:1202-9. [Crossref] [PubMed]
- Falcetta FS, Lawrie TA, Medeiros LR, et al. Laparoscopy versus laparotomy for FIGO stage I ovarian cancer. Cochrane Database Syst Rev 2016;10:CD005344. [PubMed]
- Kawka M, Fong Y, Gall TMH. Laparoscopic versus robotic abdominal and pelvic surgery: a systematic review of randomised controlled trials. Surg Endosc 2023;37:6672-81. [Crossref] [PubMed]
- Levin G, Siedhoff M, Wright KN, et al. Robotic surgery in obstetrics and gynecology: a bibliometric study. J Robot Surg 2023;17:2387-97. [Crossref] [PubMed]
- Ramirez PT, Adams S, Boggess JF, et al. Robotic-assisted surgery in gynecologic oncology: a Society of Gynecologic Oncology consensus statement. Developed by the Society of Gynecologic Oncology's Clinical Practice Robotics Task Force. Gynecol Oncol 2012;124:180-4. [Crossref] [PubMed]
- Fleming ND, Ramirez PT. Robotic surgery in gynecologic oncology. Curr Opin Oncol 2012;24:547-53. [Crossref] [PubMed]
- Krill LS, Bristow RE. Robotic surgery: gynecologic oncology. Cancer J 2013;19:167-76. [Crossref] [PubMed]
- Magrina JF, Zanagnolo V, Noble BN, et al. Robotic approach for ovarian cancer: perioperative and survival results and comparison with laparoscopy and laparotomy. Gynecol Oncol 2011;121:100-5. [Crossref] [PubMed]
- Feuer GA, Lakhi N, Barker J, et al. Perioperative and clinical outcomes in the management of epithelial ovarian cancer using a robotic or abdominal approach. Gynecol Oncol 2013;131:520-4. [Crossref] [PubMed]
- Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010;363:943-53. [Crossref] [PubMed]
- Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 2015;386:249-57. [Crossref] [PubMed]
- Coleridge SL, Bryant A, Kehoe S, et al. Neoadjuvant chemotherapy before surgery versus surgery followed by chemotherapy for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst Rev 2021;7:CD005343. [PubMed]
- Fagotti A, Ferrandina G, Vizzielli G, et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur J Cancer 2016;59:22-33. [Crossref] [PubMed]
- Onda T, Satoh T, Saito T, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur J Cancer 2016;64:22-31. [Crossref] [PubMed]
- Melamed A, Hinchcliff EM, Clemmer JT, et al. Trends in the use of neoadjuvant chemotherapy for advanced ovarian cancer in the United States. Gynecol Oncol 2016;143:236-40. [Crossref] [PubMed]
- Gueli Alletti S, Bottoni C, Fanfani F, et al. Minimally invasive interval debulking surgery in ovarian neoplasm (MISSION trial-NCT02324595): a feasibility study. Am J Obstet Gynecol 2016;214:503.e1-6. [Crossref] [PubMed]
- Pomel C, Akladios C, Lambaudie E, et al. Laparoscopic management of advanced epithelial ovarian cancer after neoadjuvant chemotherapy: a phase II prospective multicenter non-randomized trial (the CILOVE study). Int J Gynecol Cancer 2021;31:1572-8. [Crossref] [PubMed]
- Nitecki R, Rauh-Hain JA, Melamed A, et al. Laparoscopic cytoreduction After Neoadjuvant ChEmotherapy (LANCE). Int J Gynecol Cancer 2020;30:1450-4. [Crossref] [PubMed]
- Ackroyd SA, Thomas S, Angel C, et al. Interval robotic cytoreduction following neoadjuvant chemotherapy in advanced ovarian cancer. J Robot Surg 2018;12:245-50. [Crossref] [PubMed]
- Carbajal-Mamani SL, Schweer D, Markham MJ, et al. Robotic-assisted interval cytoreductive surgery in ovarian cancer: a feasibility study. Obstet Gynecol Sci 2020;63:150-7. [Crossref] [PubMed]
- Abitbol J, Gotlieb W, Zeng Z, et al. Incorporating robotic surgery into the management of ovarian cancer after neoadjuvant chemotherapy. Int J Gynecol Cancer 2019;29:1341-7. [Crossref] [PubMed]
- Psomiadou V, Prodromidou A, Fotiou A, et al. Robotic interval debulking surgery for advanced epithelial ovarian cancer: current challenge or future direction? A systematic review. J Robot Surg 2021;15:155-63. [Crossref] [PubMed]
- Zhang Y, Grant MS, Zhang X, et al. Comparing Laparotomy with Robot-assisted Interval Debulking Surgery for Patients with Advanced Epithelial Ovarian Cancer Receiving Neoadjuvant Chemotherapy. J Minim Invasive Gynecol 2021;28:1237-43. [Crossref] [PubMed]
- Van Trappen P, de Cuypere E, Claes N. Robotic surgery in early and advanced ovarian cancer: Case selection for surgical staging and interval debulking surgery. Eur J Obstet Gynecol Reprod Biol 2023;280:7-11. [Crossref] [PubMed]
Cite this article as: Levin G, Gotlieb WH. Minimally invasive cytoreduction for advanced-stage ovarian cancer—a narrative review. Gynecol Pelvic Med 2024;7:32.




